
| |||||||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�
��2008?��ɽһģ����1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���壮��ҵ�ϳ�| 20-50MPa | 500�桢����ú |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ����ʾ��
��X�Ļ�ѧʽΪ__________������________������ԡ��Ǽ��ԡ������ӡ�
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
A���¶ȡ�ѹǿ�Ի�ѧƽ���Ӱ��
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸������������
�۸ı䷴Ӧ��������ʹƽ�ⷢ���ƶ���ͼ�ұ�ʾ�������ı䣬�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������ȷ����________����������Ϊ�¶�ʱ���仯������ȷ����__________��
��2�������°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ��淴Ӧ��
___________________________________________________________________��
�ڰ�ˮ��ˮ�������c(H+)___________10��7 mol/L���>������<����=������
�۽���ˮ�������Ϻ�ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
A��c(Cl��)��c(NH4+)��c(H+)��c(OH��) B��c(Cl��)��c(NH4+)��c(OH��)��c(H+)
C��c(Cl��)��c(H+)��c(NH4+)��c(OH��) D��c(NH4+)��c(Cl��)��c(OH��)��c(H+)
������Һ��ֻ�ܽ���һ�����ʣ������ʵ������� ����������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ� ��
����������ϵ��C����ȷ�ģ�����Һ�����ʵĻ�ѧʽ�� ��
��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰ
c��HCl�� c��NH3��H2O�����>������<������=������ͬ������Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl������
��3���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����X��Y�������ʡ�XΪ��Σ�Y�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96 L����(��״��)��ͬʱ����0.3 mol X��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ�� ___________________________________��
���ڱ�״���£�ÿ����1 mol Y��ת�Ƶ��ӵ����ʵ���Ϊ___________mol��
��4����֪Һ̬NH3��H2O���ƣ�Ҳ���Է������ĵ��룬�����������ͬ��������������Һ̬NH3�ĵ��뷽��ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ��������ѧУ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ����ʾ��
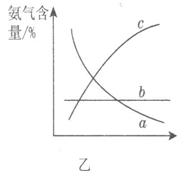
��X�Ļ�ѧʽΪ__________������________������ԡ��Ǽ��ԡ������ӡ� ��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
A���¶ȡ�ѹǿ�Ի�ѧƽ���Ӱ��
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸������������
�۸ı䷴Ӧ��������ʹƽ�ⷢ���ƶ���ͼ�ұ�ʾ�������ı䣬�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������ȷ����________����������Ϊ�¶�ʱ���仯������ȷ����__________��
��2�������°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ��淴Ӧ��
___________________________________________________________________��
�ڰ�ˮ��ˮ�������c(H+)___________10��7 mol/L���>������<����=������
�۽���ˮ�������Ϻ� ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
A��c(Cl��)��c(NH4+)��c(H+)��c(OH��) B��c(Cl��)��c(NH4+)��c(OH��)��c(H+)
C��c(Cl��)��c(H+)��c(NH4+)��c(OH��) D��c(NH4+)��c(Cl��)��c(OH��)��c(H+)
������Һ��ֻ�ܽ���һ�����ʣ������ʵ������� ����������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ� ��
����������ϵ��C����ȷ�ģ�����Һ�����ʵĻ�ѧʽ�� ��
��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰ
c��HCl�� c��NH3��H2O�����>������<������=������ͬ������Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl������
��3���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����X��Y�������ʡ�XΪ��Σ�Y�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96 L����(��״��)��ͬʱ����0.3 mol X��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ�� ___________________________________��
���ڱ�״���£�ÿ����1 mol Y��ת�Ƶ��ӵ����ʵ���Ϊ___________mol��
��4����֪Һ̬NH3��H2O���ƣ�Ҳ���Է������ĵ��룬�����������ͬ��������������Һ̬NH3�ĵ��뷽��ʽΪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ��������ѧУ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ�����ʾ��ͼ��ͼ����ʾ��


��X�Ļ�ѧʽΪ__________������________������ԡ��Ǽ��ԡ������ӡ�
��ͼ��������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ���ͬ��________��
A���¶ȡ�ѹǿ�Ի�ѧƽ���Ӱ��
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸������������
�۸ı䷴Ӧ��������ʹƽ�ⷢ���ƶ���ͼ�ұ�ʾ�������ı䣬�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������ȷ����________����������Ϊ�¶�ʱ���仯������ȷ����__________��
��2�������°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ�Ĺ����д��ڵĿ��淴Ӧ��
___________________________________________________________________��
�ڰ�ˮ��ˮ�������c(H+)___________10��7 mol/L���>������<����=������
�۽���ˮ�������Ϻ� ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
A��c(Cl��)��c(NH4+)��c(H+)��c(OH��) B��c(Cl��)��c(NH4+)��c(OH��)��c(H+)
C��c(Cl��)��c(H+)��c(NH4+)��c(OH��) D��c(NH4+)��c(Cl��)��c(OH��)��c(H+)
������Һ��ֻ�ܽ���һ�����ʣ������ʵ������� ����������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ� ��
����������ϵ��C����ȷ�ģ�����Һ�����ʵĻ�ѧʽ�� ��
��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰ
c��HCl�� c��NH3��H2O�����>������<������=������ͬ������Ϻ���Һ��c��NH4+����c��Cl�����Ĺ�ϵc��NH4+�� c��Cl������
��3���������л�ԭ�ԣ���ͭ�Ĵ������£������ͷ�����Ӧ����X��Y�������ʡ�XΪ��Σ�Y�ڱ�״����Ϊ��̬���ڴ˷�Ӧ�У���ÿ��Ӧ1���������ͬʱ��Ӧ0.75�����������ÿ��Ӧ8.96 L����(��״��)��ͬʱ����0.3 mol X��
��д�������ͷ�����Ӧ�Ļ�ѧ����ʽ�� ___________________________________��
���ڱ�״���£�ÿ����1 mol Y��ת�Ƶ��ӵ����ʵ���Ϊ___________mol��
��4����֪Һ̬NH3��H2O���ƣ�Ҳ���Է������ĵ��룬�����������ͬ��������������Һ̬NH3�ĵ��뷽��ʽΪ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com