
ij��ˮ�п��ܴ��ڵ��������£�Na+��Ag+��Ba2+��Al3+��AlO2һ��CO32����S2һ��SO32����SO42��
��ȡ����Һ�����й�ʵ�飬ʵ����̼��������£�

? ����˵����ȷ����
A������ʵ�����е�������Ƴ�������Aһ���Ǵ��������ɫ����һ����AgBr
B������ʵ�����е�������Ƴ�������B��CO2������B��Al(OH)3��ԭ��Һ��һ������Al3+
C������ʵ�����е�������Ƴ�������C��NH3������Cһ����BaCO3��������BaSO4
D��ԭ��Һ�п϶�����Na+��AlO2����S2��������ȷ���Ƿ���SO32����SO42��
C
��������
���������A. ij��ɫ��Һ�м��������HBr��Һ�����ȣ���������ɫ��������˵�����ܺ���Ag+��������Ӧ��Ag++Br-=AgBr��������ɫ����ΪAgBr��Ҳ���ܺ���S2һ��SO32����������Ӧ��2S2һ+SO32��+6H+=3S��+3H2O������ɫ����ΪS����������A��֤������CO32����S2һ��SO32��������1�֣�B. ��ҺA�м��������NH4HCO3��Һ���õ�����BΪCO2��NH4HCO3��Һ�Լ��ԣ����Բ����İ�ɫ����B����ΪAl(OH)3,��ԭ��Һ�к���AlO2-,�ڼ۹�����HBrʱ������Ӧ��4H++ AlO2-= Al3++2H2O;����C.��ҺB�м��������Ba(OH)2��Һ����������C��ΪNH3����Ӧ�ķ���ʽΪNH4++OH- NH3��+H2O������Cһ������BaCO3��������ӦHCO3��+ OH-+Ba2+=BaCO3��+H2O����ԭ��Һ�к���SO42���������C�оͺ���BaSO4��������ӦΪSO42��+Ba2+=BaSO4��.��ȷ��D.��������������֪ԭ��Һһ������AlO2-��������Al3+��AlO2һ�ᷢ�����ӷ�Ӧ�����ܹ��档���һ��������Al3+����������������S2������ȷ������ڡ�����
NH3��+H2O������Cһ������BaCO3��������ӦHCO3��+ OH-+Ba2+=BaCO3��+H2O����ԭ��Һ�к���SO42���������C�оͺ���BaSO4��������ӦΪSO42��+Ba2+=BaSO4��.��ȷ��D.��������������֪ԭ��Һһ������AlO2-��������Al3+��AlO2һ�ᷢ�����ӷ�Ӧ�����ܹ��档���һ��������Al3+����������������S2������ȷ������ڡ�����
���㣺�������ӵļ�����֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�
ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?�㽭ģ�⣩ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�
��2012?�㽭ģ�⣩ѧУ��ѧ�о�С���ij��Һ���м�����������ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3-��Cl-��MnO4-��SO42-�������еļ������ӣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪ij��ҵ��ˮ�к���N
��֪ij��ҵ��ˮ�к���N| O | - 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
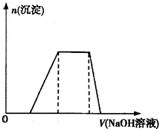
 ��ij��Һ����μ���0.5mol/L��NaOH��Һ�����ɳ��������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ�������Һ�п��ܴ��ڵ�������
��ij��Һ����μ���0.5mol/L��NaOH��Һ�����ɳ��������������NaOH��Һ�����֮��Ĺ�ϵ����ͼ��ʾ�������Һ�п��ܴ��ڵ�������| A��ֻ��Al2+ | B����Mg2+��Al3+ | C����H+��Mg2+��Al3+ | D����NH4+��Mg2+��Al3+ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com