
��10�֣�X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81��8����
��1��1��Y������Ӧ���� ����ԭ�ӡ�
��2��X�ķ���ʽ�� ��
��
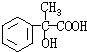
��3��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
��д��A�Ľṹ��ʽ ��
��E�� F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| Ũ���� |
| �� |
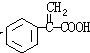 +H2O
+H2O
| Ũ���� |
| �� |
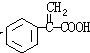 +H2O
+H2O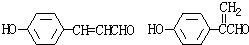

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 molY��1mol CH3CH2OH��X��Y����Է���������������200����ȫȼ�ն�ֻ����CO2��H2O����x������̼����Ԫ���ܵ������ٷֺ���ԼΪ81��8����
��1��1��Y������Ӧ���� ����ԭ�ӡ�
��2��X�ķ���ʽ�� ��
��3��G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
 |
��д��A�Ľṹ��ʽ ��
��E��F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
i�������ڳ��˱��������������ṹ���ұ�������2����λȡ������
ii��һ�������£������ʼ�����������Һ����������ӦҲ�ܺ�FeCl3��Һ������ɫ��Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y���Ƿ����廯�����Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1 molX
ˮ��õ�1 mol Y��1 mol CH3CH2OH��X��Y�ķ�������������200����ȫȼ�ն�ֻ����
CO2��H2O����X������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8%��
(1) X��Y������֮��Ϊ ��
(2)1��Y������Ӧ���� ����ԭ�ӡ�
(3) X�ķ���ʽ��_________ ______��
(4) G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ��÷�����A�ϳ�G·�����£�
�� д��A�Ľṹ��ʽ
�� E��F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�� д�����з�������������F��ͬ���칹��Ľṹ��ʽ��
���������ڳ��˱�����������״�ṹ���ұ�������2����λȡ������?
����һ�������£������ʼ�����������Һ����������Ӧ���ܺ�FeCl3��Һ������ɫ��Ӧ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y���Ƿ����廯����,��Ϊ����ʳ���㾫���㷺���ڻ�ױƷ���ǹ�����ζƷ�С�1mol Xˮ��õ�1 mol Y��1 mol CH3CH2OH��X��Y�ķ�������������200����ȫȼ�ն�ֻ����CO2��H2O����X������̼����Ԫ���ܵ������ٷֺ���ԼΪ81.8%��
(1) X��Y������֮��Ϊ_____________��
(2)1��Y������Ӧ����________����ԭ�ӡ�
(3) X�ķ���ʽ��_________ ______��
(4) G��X��Ϊͬ���칹�壬�Ҿ�����ͬ�Ĺ����ţ�E�������ԣ��÷�����A�ϳ�G·�����£�
�� д��A�Ľṹ��ʽ
�� E��F�ķ�Ӧ������ ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�� д��D������Cu(OH)2��Ӧ�Ļ�ѧ����ʽ��
�� д��E�������ɸ߷��ӻ�����ķ�Ӧ����ʽ��
�� д�����з�������������F��ͬ���칹��Ľṹ��ʽ�� ��
���������ڳ��˱�����������״�ṹ���ұ�������2����λȡ������?
����һ�������£������ʼ�����������Һ����������Ӧ���ܺ�FeCl3��Һ������ɫ��Ӧ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com