
��14�֣�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ�ֻ�����DA���۵�Ϊ800�棬DA����ˮ��Ӧ�ų�����������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ���������������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ ��
��14�֣���1���������ڣ�IIIA�壻 ��2��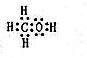
��3��H2O2 H++HO
H++HO ��BaO2+H2SO4=BaSO4��+H2O2��
��BaO2+H2SO4=BaSO4��+H2O2��
��4��Cu+ 2H++H2O2=Cu2++2H2O
��5��56L
��6��3Na2O2+6Fe2++6H2O=4Fe(OH)3��+6Na++2Fe3+
��������Ԫ���ƶϣ�A��B��W��D��EΪ������Ԫ�أ���ԭ����������������A��W���γ�����Һ̬������A2W��A2W2֪AΪH��WΪO��������֪DΪNa��EԪ�ص���������������������ȣ���֪ΪAl��������Ԫ��������֮��Ϊ39��֪BΪCԪ�أ�
��2����A��B��W����Ԫ����ɵ�18����������H��C��O�����ߵ������ֱ�Ϊ1��6��8��������Ϊ15��ֻ���ټ�������ԭ�ӣ���18������Ӧ��ΪCH4O��Ҳ���Ǽ״���
��3������ǿ�������ᣬ��֪��BaO2+H2SO4=BaSO4��+H2O2
��5���ɣ� 2Na +H2 =2NaH�� NaH + H2O = NaOH + H2����
2Al+2NaOH+2H2O =2NaAlO2+3H2��
֪��2Al+2NaH+4H2O =2NaAlO2+5H2����������2.5molH2��Ҳ����56L��
��5��2Na2O2+2H2O=4Na++4OH�� + O2��
4Fe2+ +O2 +2H2O=4Fe3++4OH��
��ʽ��ӣ�2Na2O2+4Fe2+ +4H2O=4Na++8OH�� + 4Fe3+
��ʽ�ұ�OH��Ӧ��ȫ������ Fe(OH)3����Fe3++3OH�� = Fe(OH)3��
2Na2O2+4Fe2+ +4H2O=4Na++8/3Fe(OH)3�� + 4/3Fe3+
�����ٳ�3/2����3Na2O2+6Fe2++6H2O=4Fe(OH)3��+6Na++2Fe3+



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 HO2-+H+
HO2-+H+ HO2-+H+
HO2-+H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
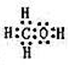
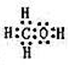
 H++HO2-
H++HO2- H++HO2-
H++HO2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2�ͺ�ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���Ȼ���DA���۵�Ϊ800��DA����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ�����ȫ������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡǦɽһ�е���У��һ��ѧ��������ѧ�Ծ����������� ���ͣ������
��14�֣�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ�ֻ�����DA���۵�Ϊ800�棬DA����ˮ��Ӧ�ų�����������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ���������������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ȱ������и�����һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
�Իش�
��1��EԪ�������ڱ��е�λ��Ϊ���������������������������� ����������
��2�� ��A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ �� ������������ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ������������������������ �����������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ�������������������������� ������
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ���������������������������������� ������
��5�� Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���⻯��DA���۵�Ϊ800�档DA����ˮ��Ӧ������������1mol DA��1 mol E���ʻ�ϼ���������ˮ����ַ�Ӧ�������������������� ������״���£���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com