
��16�֣� ��1����֪��
��1����֪��
O2 (g) = O+2(g) + e- 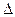 H1=" 1175.7" kJ��mol-1
H1=" 1175.7" kJ��mol-1 PtF6(g) + e- = PtF6-(g)
PtF6(g) + e- = PtF6-(g)
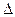 H2=" -" 771.1 kJ��mol-1
H2=" -" 771.1 kJ��mol-1 O2+ PtF6-(s) = O2+(g) + PtF6-
O2+ PtF6-(s) = O2+(g) + PtF6- 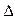 H3="482.2" kJ��mol-1
H3="482.2" kJ��mol-1  ��ӦO
��ӦO 2��g��+ PtF6 (g) = O2+PtF6- (s)
2��g��+ PtF6 (g) = O2+PtF6- (s) 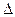 H="_____________" kJ��mol-1
H="_____________" kJ��mol-1
��2����C��S�γɵ�Һ̬������CS2��0.2mol/l CS2��O2����ȫȼ�գ�����������̬�����298Kʱ�ų�����215k J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
��3����֪��������0.1mol/L��ij��H2A��pH=4�������Ϊ �ᣨ�ǿ������������H2A�ĵ��뷽��ʽΪ ������Һ����ˮ�������c(H+)= ��
��4��һ���¶��£������a������ b�����
�ٵ�����������ʵ���Ũ����ͬʱ��c(H+)��a b�����������������=������ͬ����
����pH��ͬ�������ͬ����������Һ�м�����������ۣ���Ӧ����ʱ����H2���������a b��
�۽�pH��ͬ�������ͬ����������Һ�ֱ��ˮϡ��100����������Һ��pHֵ��a b

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?բ������ģ����ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
��2013?բ������ģ����ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺| C(H2)?C(CO) |
| C(H2O) |
| ���� | H2 | CO | CH3OH |
| Ũ�ȣ�mol/L�� | 0.2 | 0.1 | 0.4 |
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ����У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
��1����֪��O2 (g)= O2�� (g)+e�� ��H1= +1175.7 kJ��mol��1
PtF6(g)+ e��= PtF6��(g)???? ��H2= - 771.1 kJ��mol��1
O2+PtF6��(s)=O2+(g)+PtF6�� (g)?? ��H3=+482.2 kJ��mol��1
��Ӧ��O2��g��+ PtF6 (g) = O2+PtF6(s)����H=_____ kJ��mol-1��
��ͼΪ�ϳɰ���Ӧ��ʹ����ͬ�Ĵ�������ͬ�¶Ⱥ�ѹǿ�����½��з� Ӧ����ʼʱN2��H2�������Ϊ1:3ʱ��ƽ�������а������������

�� ��һ�����¶��£������������ܱ������г��뵪������������������Ӧ��������˵����Ӧ�ﵽƽ��״̬����??????? ��
a����ϵ��ѹǿ���ֲ���?? ???? b�����������ܶȱ��ֲ���
c��N2��H2�������Ϊ1:3????? d����������ƽ��Ħ����������
���ֱ���vA��NH3����vB��NH3����ʾ�ӷ�Ӧ��ʼ��ƽ��״̬A��Bʱ�ķ�Ӧ���ʣ���vA��NH3��??? vB��NH3��������>������<������=�������÷�Ӧ�ĵ�ƽ�ⳣ��kA ??? kB������>������<������=��������250 ����1.0��104kPa�´ﵽƽ�⣬H2��ת����Ϊ????? %������������С�����һλ����
��3��25��ʱ����a mol NH4NO3����ˮ����Һ�����ԣ�ԭ��???????????????????????? ?????????? �������ӷ���ʽ��ʾ���������Һ�м���bL��ˮ����Һ�����ԣ������Ӱ�ˮ��Ũ��Ϊ?????????? mol/L���ú�a��b�Ĵ���ʽ��ʾ��NH3��H2O�ĵ���ƽ�ⳣ��ΪKb=2��10-5��
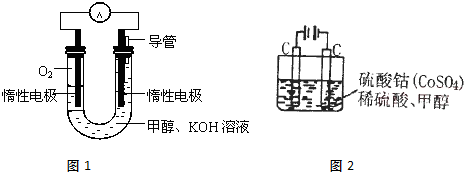
��4����ͼ��ʾ��װ����Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ����ʵ�������϶�ͭ�����һ��ʱ���װ��������Һ��pH ???? �����������������С����������������a���缫��Ӧ����ʽΪ????????????????? ������ƽ�������װ���������������仯��25.6g����Һ������ͭ��ʣ�ࣩ����װ���������������ļ���????? L����״���£���

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com