
��������̼�Ƿ�����ˮ����ʱ������������Ʒ�Ӧ�����������ʵ�������ͨ������ʵ�����˵����
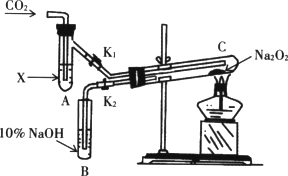
�ٰ�ͼװ�ã��ڸ�����Թ�C��װ��Na2O2����ͨ��CO2֮ǰ��Ӧ�����õ��ɼ�(K1��K2)�гֺã���Ŀ��Ϊ________��
���Թ�A�ڵ��Լ�XӦ��________ʱ�����ɼ�K1��K2�������Թ�CԼ�����Ӻ������ǵ�Сľ�������Թ�B��Һ���ϣ��ɹ۲쵽�����ǵ�Сľ�����ܾ��ҵ�ȼ�գ����Թ�C�ڵ���ɫ��ĩδ�����仯�������õĻ�ѧ������________��
���Թ�A���Լ�ΪCO2����ˮ��Һʱ����������ͬ�ڣ�ͨ��________���������֤��Na2O2�볱ʪ��CO2�ܷ�Ӧ�ҷų�O2��
��CO2��Na2O2��Ӧ����Ҳ����ʾ��ԭ�ӷ�������֤������������з�Ӧ��Na2O2��C18O2��H218O________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
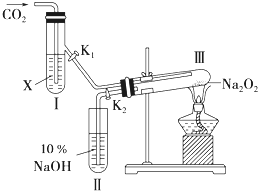 ��ͼ��ʾ����������̼�Ƿ�����ˮ����ʱ��������������Ʒ�Ӧ������������ͨ������ʵ�����֤����
��ͼ��ʾ����������̼�Ƿ�����ˮ����ʱ��������������Ʒ�Ӧ������������ͨ������ʵ�����֤����18 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2012�������ѧһ�ָ���ѵ������4�¡���1�����Ƽ��仯����(³�ư�) ���ͣ�058
��ͼ��ʾ����������̼�Ƿ�����ˮ����ʱ��������������Ʒ�Ӧ������������ͨ������ʵ�����֤����
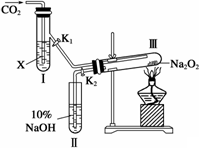
(1)��ͼװ�ã��ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ��Ӧ���Ƚ�����(K1��K2)�رպã�Ŀ�ĺ��ڣ�
________��
(2)�Թܢ��ڵ��Լ�X��________ʱ������K1��K2�������Թܢ�Լ5���Ӻ������ǵ�Сľ�������Թܢ��Һ���ϣ��ɹ۲쵽�����ǵ�Сľ�����ܾ��ҵ�ȼ���������Ң��ڵ���ɫ��ĩδ�����仯�������õĻ�ѧ������________��
(3)�Թܢ����Լ�ΪCO2����ˮ��Һʱ����������ͬ(2)��ͨ��________��������֤��Na2O2�볱ʪ��CO2�ܷ�Ӧ�ҷų�O2��
(4)CO2��������Ʒ�Ӧ����Ҳ����ʾ��ԭ�ӷ�������֤������������з�Ӧ����ʽ��
________Na2O2��________C18O2��________H![]() O��________
O��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��������̼�Ƿ�����ˮ����ʱ������������Ʒ�Ӧ����
�������ʵ�������ͨ������ʵ�����֤����
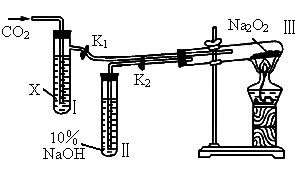
(1)��ͼװ��(�̶��Թܢ�����̨��ȥ)���ڸ�����Թܢ���װ��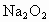 ����ͨ��
����ͨ��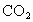 ֮ǰ��Ӧ�����õ��ɼ�(
֮ǰ��Ӧ�����õ��ɼ�(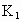 ��
��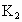 )�гֺã�Ŀ����
)�гֺã�Ŀ����
________________________��
(2)�Թܢ��ڵ��Լ�X��________�ģ����ɼ�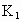 ��
��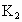 �������Թܢ�Լ5min�������ǵ�ľ�������Թܢ��Һ���ϣ��ɹ۲쵽�����ǵ�ľ��ȼ�գ����Թܢ��ڵ���ɫ��ĩû�����仯�������õĻ�ѧ������________________________��
�������Թܢ�Լ5min�������ǵ�ľ�������Թܢ��Һ���ϣ��ɹ۲쵽�����ǵ�ľ��ȼ�գ����Թܢ��ڵ���ɫ��ĩû�����仯�������õĻ�ѧ������________________________��
(3)�Թܢ����Լ�Ϊ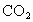 ����ˮ��Һʱ����������ͬ(2)��ͨ��________���������֤��
����ˮ��Һʱ����������ͬ(2)��ͨ��________���������֤��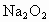 �볱ʪ��
�볱ʪ��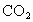 �ܷ�Ӧ�ҷų�
�ܷ�Ӧ�ҷų�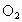 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
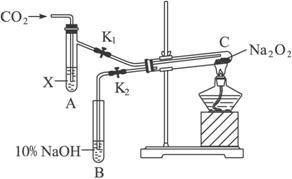
��1����ͼװ�ã��ڸ�����Թ�C��װ��Na2O2����ͨ��CO2֮ǰ��Ӧ�����õ��ɼУ�K1��K2���гֺã���Ŀ��Ϊ___________________________��
��2���Թ�A�ڵ��Լ�XӦ��______________ʱ�����ɼ�K1��K2�������Թ�CԼ�����Ӻ������ǵ�Сľ�������Թ�B��Һ���ϣ��ɹ۲쵽�����ǵ�Сľ�����ܾ��ҵ�ȼ�գ����Թ�C�ڵ���ɫ��ĩδ�����仯����ó��Ľ�����___________________________��
��3���Թ�A���Լ�ΪCO2����ˮ��Һʱ����������ͬ��2����ͨ��____________________���������֤��Na2O2�볱ʪ��CO2�ܷ�Ӧ�ҷų�O2��
��4��CO2��Na2O2��Ӧ����Ҳ��ͨ��ʾ��ԭ�ӷ�������֤������������з�Ӧ��
Na2O2+C 18O2+H218O====_________________________________________________��
��5����ͼʵ���������֤Na2O2��CO2�ķ�Ӧ���۲쵽��ʵ�������ǣ�����Na2O2����ȼ�ա��Ӹ�ʵ����Եó�������ۣ�����д���ɸ�ʵ��ó����������ۡ�
����1��______________________________________��
����2��______________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(����̽��)��ͼ��ʾ����������̼�Ƿ�����ˮ����ʱ��������������Ʒ�Ӧ������������ͨ������ʵ�����֤����
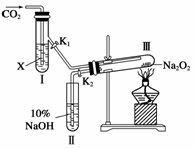
(1)��ͼװ�ã��ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ��Ӧ���Ƚ�����(K1��K2)�رպã�Ŀ�ĺ��ڣ�
________________________________________________________________________
________________________________________________________________________��
(2)�Թܢ��ڵ��Լ�X��____________ʱ������K1��K2�������Թܢ�Լ5���Ӻ������ǵ�Сľ�������Թܢ��Һ���ϣ��ɹ۲쵽�����ǵ�Сľ�����ܾ��ҵ�ȼ���������Ң��ڵ���ɫ��ĩδ�����仯�������õĻ�ѧ������
________________________________________________________________________��
(3)�Թܢ����Լ�ΪCO2����ˮ��Һʱ����������ͬ(2)��ͨ��
____________________________________________________________________
________________________��������֤��Na2O2�볱ʪ��CO2�ܷ�Ӧ�ҷų�O2��
(4)CO2��������Ʒ�Ӧ����Ҳ����ʾ��ԭ�ӷ�������֤������������з�Ӧ����ʽ��
__Na2O2��__C18O2��__H![]() O�D��
O�D��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com