��2013?����һģ����ͼΪԪ�����ڱ���һ���֣������Ԫ�آ�һ���ڱ��е�λ�ã��ش���������
| �� |
��A |
|
0 |
| ���� |
| 1 |
�� |
��A |
��A |
��A |
��A |
��A |
����A |
|
| 2 |
|
|
|
�� |
�� |
�� |
�� |
|
| 3 |
�� |
|
�� |
|
|
|
�� |
|
��1��Ԫ�آ��γɵļ������ӵĽṹʾ��ͼΪ
��Ԫ�آܡ��ޡ����γɵļ����ӵİ뾶�ɴ�С��˳����
O2-��Na+��Al3+
O2-��Na+��Al3+
�������ӷ��ű�ʾ����
��2��Ԫ�آۺܿ͢����γɶ��ֻ�������л������Ϊ��Щ����������Է���������С�ģ��������������Ԫ�آܵĵ��ʰ����ʵ���֮��Ϊ4��1ͨ�뺬������Ԫ�آ١��ܺ͢���ɵĻ������ҵ�ˮ��Һ�У�����ǡ����ȫ��Ӧ�����ɵ���ֻ��һ�֣��䷴Ӧ�Ļ�ѧ����ʽΪ
4NO+O2+4NaOH=4NaNO2+2H2O
4NO+O2+4NaOH=4NaNO2+2H2O
��
��3��Ԫ�آں͢���ɵ�һ�ֻ�������Ԫ�آܵĵ��ʺͻ������ҵ�ˮ��Һ��һ�������¿��γ�ȼ�ϵ�أ�д����ȼ�ϵ�ظ����ĵ缫��Ӧʽ
CO+4OH-+2e-=CO32-+2H2O
CO+4OH-+2e-=CO32-+2H2O
��
��4���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
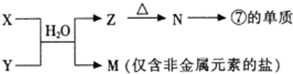
��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ
Al3++3NH3?H2O=Al��OH��3��+3NH4+
Al3++3NH3?H2O=Al��OH��3��+3NH4+
��
��N���ߵĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ
��
��M��ˮ��Һ�����ԣ������ӷ���ʽ������ԭ��
NH4++H2O?NH3?H2O+H+
NH4++H2O?NH3?H2O+H+
��




 ��
�� ��O2-��Na+��Al3+��
��O2-��Na+��Al3+��

