
������������ɫ����ˮ����ζ��Һ�壬�е�77.2�棬ʵ����ij����ȡ���ñ�����14.3mL��95���Ҵ�23mL�����õ�Ũ���ᡢ����̼�����Լ��������Ҵ���ϳ���ˮ������Ȼ�����Һ����Ҫ��������ͼ��ʾ��
ʵ�鲽���ǣ�
������A��������ƿע�������Ҵ���Ũ�����ҡ�ȣ��ٽ�ʣ�µ������Ҵ��ͱ�����ע���Һ©������ã���ʱ��Һ©����������������ʵ�����ԼΪ5��7��
�ڼ�����ԡ����Լ135��145�森
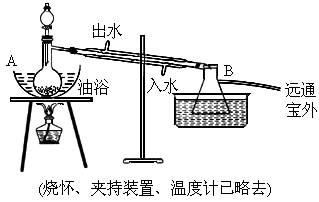
�۽���Һ©���е�Һ�建������������ƿ����ڼ����ٶ�ʹ���������ٶ�������ٶȴ�����ȣ�ֱ��������ɣ�
�ܱ�����ԡ�¶�һ��ʱ�䣬��������Һ��Һ��ֹͣ���ȣ�
��ȡ��B�е���ƿ����һ��������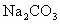 ��Һ����������εؼӵ����Һ��ӱ�ҡ�����������ݲ���Ϊֹ��
��Һ����������εؼӵ����Һ��ӱ�ҡ�����������ݲ���Ϊֹ��
���ݵ�Һ������Һ����ȡˮ�㣮
���ñ���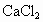 ��Һ(����)���뵽��Һ©���У�ҡ��һ��ʱ����ã��ų�ˮ��(��Һ)��
��Һ(����)���뵽��Һ©���У�ҡ��һ��ʱ����ã��ų�ˮ��(��Һ)��
���Һ©����õ����dz����������������Ʒ��
�Իش�
(1)ʵ�����������Ҫ������___________________________��
(2)�ù����Ҵ�����ҪĿ����___________________________��
(3)�ñ���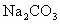 ��Һϴ�Ӵ�����Ŀ����___________________�������NaOHŨ��Һ����
��Һϴ�Ӵ�����Ŀ����___________________�������NaOHŨ��Һ����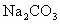 ������ĺ����_____________________��
���������_____________________��
(4)�ñ���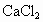 ��Һϴ�Ӵ�����Ŀ����__________________��
��Һϴ�Ӵ�����Ŀ����__________________��
(5)Ϊʲô����Ҫʹ������������ٶȴ������?_________________��
(6)����ʾ�Ĵ����ﻹ�е�������________________��
(7)B����ƿΪʲôҪ�����ܰ������ͳ�����?___________________��
|
(1) ʵ�����������Ҫ�����Ǵ����ú���ˮ����(2) �ù����ҵ���ҪĿ����ʹ������Ӧ���������������ķ����ƶ���������������IJ���(3) �ñ���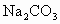 ��Һϴ�Ӵ�����Ŀ���dz�ȥ���ᣮ�����NaOHŨ�ܴ��� ��Һϴ�Ӵ�����Ŀ���dz�ȥ���ᣮ�����NaOHŨ�ܴ���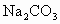 �����ʹ������������ˮ���ʹʵ��ʧ�� �����ʹ������������ˮ���ʹʵ��ʧ��
(4) �ñ��� Һϴ�Ӵ�����Ŀ���dz�ȥ�Ҵ� Һϴ�Ӵ�����Ŀ���dz�ȥ�Ҵ�
(5) �ò���������������ʱ�������(6) �����ﻹ�е�������ˮ(7) ��ʵ���в����� ���ж������ų����⣬Ҳ����������������Һ��������ƿ����ʹ��ƿ�е�ѹǿ����һ�� ���ж������ų����⣬Ҳ����������������Һ��������ƿ����ʹ��ƿ�е�ѹǿ����һ�� |


 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
| ���� |
| ���� |
| A | B |
| �ٷ���ʽΪC3H8O�� ����Cu�������´������IJ��� ���ܷ���������Ӧ |
����C��H����Ԫ����ɣ� �����ģ��Ϊ��  |
| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
 +HNO3��Ũ��
+HNO3��Ũ��| ŨH2SO4 |
| �� |

 +HNO3��Ũ��
+HNO3��Ũ��| ŨH2SO4 |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
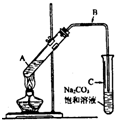 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O| ����� | ���� | ��Ӧ���� | C�б���̼������Һ������߶� |
| �� | 2mL98%Ũ���� | 20��ʱ��Һ������ɫ���淴Ӧ���У���Һ��ɫ������ɺ�ɫ�����������ݣ� | 2.10cm |
| �� | 2mL14mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.14cm |
| �� | 2mL10mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� | 2.16cm |
| �� | 2mL7mol?L-1���� | ��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ�����ݣ� | 2.00cm |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��1������ˮ��������������________��
����ˮ����������ͨ��ֱ��ˮ��������ȣ����е��ŵ���________��������a������ȴˮ��________�����ڻ���ڣ���
��2��������ƿ�е�Һ��Ӧ�ã������������ƣ���
��3��������ƿ��ȡ�����������м���������Ƭ������________��Һ�����Ժ���ɫ��ԭ����________��
��4��������ƿ��ȡ�����������еμӼ��θ�����ص�������Һ������ˮ���е��Ϻ�ɫ����ȥ��ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
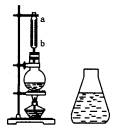
��ͼ���Ʊ�����������ʵ��װ�ã�Բ����ƿ��Ӧװ��Ũ���ᡢ���ᡢ�Ҵ��ͷ�ʯ����ƿ�Ͻ�������ˮ��������С�ļ��ȣ�ʹ��ƿ�ڵ�Һ�岻���ڣ���Ӧһ��ʱ�����ƿ��Һ�嵹��װ�б���̼������Һ����ƿ�У�ƿ�е���ɫҺ��ֳ����㡣
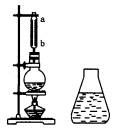
��1������ˮ��������������________��
����ˮ����������ͨ��ֱ��ˮ��������ȣ����е��ŵ���________��������a������ȴˮ��________�����ڻ���ڣ���
��2��������ƿ�е�Һ��Ӧ�ã������������ƣ���
��3��������ƿ��ȡ�����������м���������Ƭ������________��Һ�����Ժ���ɫ��ԭ����________��
��4��������ƿ��ȡ�����������еμӼ��θ�����ص�������Һ������ˮ���е��Ϻ�ɫ����ȥ��ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������á��߶���ѧ(��) ���ͣ�013
|
�������ˮ�ܼ���ˮһ�����Է����������룬�Ӷ�����һ���ĵ����ԣ��磺Һ̬BrF3�У�2BrF3 | |
| [����] | |
A�� |
������Ӧ��ͭ����ֻ�������� |
B�� |
������Ӧ�в���������ΪNO |
C�� |
������Ӧ�е�����ͭ�ǻ�ԭ���� |
D�� |
������Ӧ��N2O4���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com