
����Ŀ��ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����K2Cr2O7��ʵ���ҶԺ�����Һ������Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72���������������ù������£�
��֪����Cr(OH)3+OH��=CrO2��+2H2O�� ��2CrO2��+3H2O2+2OH��=2CrO42��+4H2O��M(Cr)=52
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��

��1�����˹�����Ҫ��ʱ�۲�����ƿ��Һ��߶ȣ�����ﵽ֧�ܿ�λ��ʱӦ���еIJ���Ϊ_____________________________��
��2����Һ���ữǰ�����м��ȵ�Ŀ����____________________________��
��ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ����________________________��
��3���±���������ʵ��ܽ�����ݣ�
���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�����ܽ�����ݣ�����pHѡ����Լ���__________________��
A��ϡ���� B��ϡ���� C��ϡ����
������������������______________________����____________________��
��4����ȡ��Ʒ�ظ��������4.000g���250mL��Һ��ȡ��25.00mL����ƿ�У�����10mL 2 mol��L��1H2SO4�������⻯�ƣ����Ļ�ԭ����ΪCr3+�������ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.2400 mol��L��1Na2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62������
�� ��ʵ���й���ȥNa2S2O3����Һ30.00mL�����ò�Ʒ�����ظ���صĴ���Ϊ__________________________����ʽ�����㣬�������������������ʲ����뷴Ӧ����
�� ��װNa2S2O3��Һ�ĵζ����ڵζ�ǰ�����ݵζ���û�����ݣ���õ��ظ���صĴ��Ƚ���______________________���ƫ�ߡ�����ƫ�͡������䡱����
���𰸡� �ε�����ƿ�ϵ���Ƥ�ܣ�������ƿ�Ͽڵ�����Һ��ȥH2O2 ��ȥ�����������ʣ������پ������ʧ B ������Ũ���� �ᾧ ���ȹ��� ![]() ��100% ƫ��
��100% ƫ��
��������ʵ���ҶԺ�����Һ(����Cr3+��Fe3+��K+��SO42-��NO3-������Cr2O72-)���գ��ȼ�KOH��Cr3+��Fe3+ת�����������������������м�˫��ˮ��KOH����Cr(OH)3ת��ΪCrO42-������������CrO42-ת��ΪCr2O72-��ͨ������Ũ�������˵õ�K2Cr2O7��
(1)ʵ��ʱ��������ƿ��Һ��߶ȿ�ﵽ֧�ܿ�λ��ʱ��Ϊ��ֹҺ���������װ��Ӧ�õ�������Һ�壬��������������ǣ��ε�����ƿ�ϵ���Ƥ�ܣ�������ƿ�Ͽڵ�����Һ���ʴ�Ϊ���ε�����ƿ�ϵ���Ƥ�ܣ�������ƿ�Ͽڵ�����Һ��
(2)H2O2���ȶ��������ֽ⣬����ͨ����������ȥH2O2��K2Cr2O7����ˮ�е��ܽ�Ƚ�С����������ˮϴ��K2Cr2O7���ܳ�ȥ���������������ʣ����ܼ�СK2Cr2O7����ģ��ʴ�Ϊ����ȥH2O2����ȥ���������������ʣ���СK2Cr2O7����ģ�
(3)K2Cr2O7����ǿ�����ԣ��ܹ��������ᣬ���������ᣬ����ص��ܽ�Ƚϴ�������K2Cr2O7���룬��ѡϡ�����ữ����ѡB�����ݱ������ݿ�֪�¶Ƚϸ�ʱK2Cr2O7���ܽ�Ƚϴ��������ʵ��ܽ�Ƚ�С������Ũ��ʹ����ת��Ϊ�����������¶Ƚϸ�ʱK2Cr2O7���������壬����Ҫ���ȹ��ˣ��ʴ�Ϊ��B�������ᾧ�����ȹ��ˣ�
(4)���ɷ�ӦCr2O72-+6I-+14H+=2Cr3++3I2+7H2O��I2+2S2O32-=2I-+S4O62-�ɵ÷�Ӧ�Ĺ�ϵʽΪCr2O72-��3I2��6S2O32-�����ݹ�ϵʽ���㣬
Cr2O72-��3I2��6S2O32-
1mol 6mol
n 0.2400��30��10-3mol
��250ml���ظ���ص����ʵ���Ϊn=![]() ��10�������ò�Ʒ���ظ���ش���Ϊ
��10�������ò�Ʒ���ظ���ش���Ϊ![]() ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ�� ![]() ��100%��
��100%��
��װNa2S2O3��Һ�ĵζ����ڵζ�ǰ�����ݵζ���û�����ݣ������V(��)ƫ��������Na2S2O3�����ʵ���ƫ���ظ���ص����ʵ���ƫ�����õ��ظ���صĴ��Ƚ�ƫ�ߣ��ʴ�Ϊ��ƫ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��δ������Դ���ص�����Դ�ḻ,��ʹ��ʱ�Ի�������Ⱦ����Ⱦ��С,�ҿ������������з���δ������Դ�����У�������
����Ȼ������ú���ۺ��ܡ���ʯ�͡���̫���ܡ����������ܡ��߷��ܡ�������
A.�٢ڢݢޢ�B.�ݢޢߢ�
C.�ۢݢޢߢ�D.�ۢܢݢޢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ��״���£�CO��һ����ɫ����ζ���ж������壬������ˮ�����ᡢ�����Һ������Ӧ���ƾ���ƿ�����������Դ����ȷ������ͼ��ʾ��װ�ý���ʵ�飬������֤ij�������ijɷ���CO2��CO��ÿ��װ������һ��������ش��������⣺
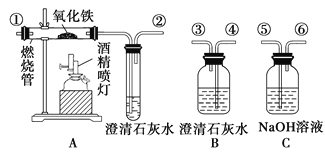
��1������װ�õ��ܿڵ�˳���������__________________��β����������ܽӿڴ�������
��2��֤��ԭ���������CO2���ڵ�ʵ��������__________________________________��
֤��CO���ڵ��йط�Ӧ�Ļ�ѧ����ʽ��__________________________________________��
��3����ͬѧ�������BӦ��ʹ��һ�Σ�����Ϊ�е�����________��������������û�����������������ɣ�
_________________________________________________________________��
��4����ʵ��β�������ķ�����________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
A ��ȡ��Һ�� B �ᾧ�� C ��Һ�� D ���� E ���˷� F ������
��_____���뱥��ʳ��ˮ��ɳ�ӵĻ���
��_____����ˮ�����͵Ļ���
��_____�������Ȼ�̼���е�Ϊ 76.75�棩�ͼױ����е�Ϊ 110.6�棩�Ļ���
��_____�ӵ��ˮ��Һ����ȡ�⡣
��_____������غ��Ȼ��ƵĻ��Һ�л������ء�
����ͼ��ijͬѧ��Ƶ�����װ��ͼ
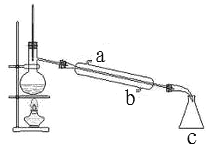
�ٽ�ˮ����_____��a �� b����
��װ�� c ��������_____��
��������ƿ�г������ʯ�����Ƭ����Ŀ����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ������Ĺ���������
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��ش���
(1)�����Ҵ���Ŀ����__________________________________________��
(2)֤����֬��ȫ��Ӧ�ķ�����_____________________________________________________��
(3)��Ӳ֬�������Ϊ����д��������Ӧ�ķ���ʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ȼ���ױ����ж��� ��ѧ���ʣ������������װ������Σ�վ����ǩ�������� �е������У���ǩ�����˵���
ѡ�� | A | B | C | D |
���ʵĻ�ѧʽ | �������� | ������ | ���Ȼ�̼ | �̻����� |
Σ�վ����ǩ |
|
|
|
|
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ���ǣ�������
�ٱ�״���£�0.2 mol�κ����ʵ������Ϊ4.48 L������1 mol��������Ϊ22.4 L������һ�����ڱ�״���¡��۱�״���£�1 L HCl��1 L H2O�����ʵ�����ͬ���ܱ�״���£�1 g H2��14 g N2�������ͬ����28 g CO�����Ϊ22.4 L�����������ʵ����ʵ�����ͬ���������ڱ�״���µ����Ҳ��ͬ������ͬ��ͬ���ʱ���������ʵ����ʵ���Խ����ѹǿԽ��ͬ��ͬѹ�£�������ܶ����������Է�������������
A. �٢ڢۢ� B. �ڢۢޢߢ� C. �ݢޢߢ� D. �ܢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ����Ԫ�����ڱ��е�λ�ã�ȡ����Ԫ��ԭ�ӵ�(����)
A.���ԭ�������ͺ˵����B.���Ӳ�����������
C.���Ӳ���������������D.���Ӳ����ͺ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯�����ڹ�ũҵ����������Ҫ�����á�
��1����֪����C(s)+O2(g)=CO2(g) ��H1=-393.5kJ/mol��
��C(s)+CO2(g)=2CO(g) ��H2=+172.5kJ/mol
��4Fe(s)+3O2(g)=2Fe2O3(s) ��H3=-1651.0kJ/mol
CO��ԭFe2O3���Ȼ�ѧ����ʽΪ__________________________________________��
��2����¯���������ĸ�¯���к���CO��H2��CO2�����壬��CO��H2�ڴ��������ºϳɼ״����Ǽ�����Ⱦ����Լ��Դ���¾ٴ룬��Ӧԭ����CO(g)+2H2(g)![]() CH3OH(g) ��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��
CH3OH(g) ��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��
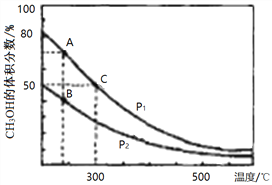
������ͼA��B��C�����У�ѡ����Ӧ�±���������С�ĵ㡣
��Ӧ���� | ƽ�ⳣ��K | ƽ��ת������ |
_________ | _________ | _________ |
����300��ʱ����C��ƽ����ϵ���ٳ���0. 5molCO��1.0molH2��0.5mol��CH3OH���÷�Ӧ��_________������У���������Ӧ�������淴Ӧ���������ƶ�������
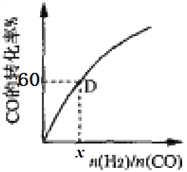
��һ���¶��£�CO��ת��������ʼͶ�ϱ�[n(H2)/n(CO)]�ı仯��ϵͼ��ʾ�����D��������ת����Ϊ40%����x=_____________��
��3�����Ȼ�����һ����Ҫ�Ļ��������������ʴ��·�塣ij��ʴ��Һ�к���0.5mol��L-1Fe3+��0.26mol��L-1��Cu2+����ʹFe3+��ȫ����[c(Fe3+)��4��l0-5]��Cu2+�����������������ҺpH�ķ�ΧΪ_________��[KspCu(OH)2=2.6��l0-19��KspFe(OH)3=4��l0-38]
��4��Ī���Σ�����ˮ����������茶��壬��һ����Ҫ�Ļ���ԭ�ϣ��ڿ����л����绯����������֤��һƿ���õ�Ī�����Ѿ�������������Ҫ����ʵ������ǣ�ȡ������Ʒ��������ˮ�ܽ⣬����Һ�ֳ����ݣ�______________________________________����֤������Ʒ�Ѳ���������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com