
(16��) ͼ����ʵ�����г������Ʊ����������װ��:
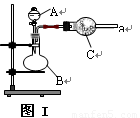
(1) ��ͬѧ��ͼ��װ�á�ͭ��Ũ�����Ʊ����ռ������NO2���壺
�� B�з�Ӧ�Ļ�ѧ����ʽ_____________________________��
�� �ռ�NO2����ķ��� ��
�� ���ռ���NO2����ƿ�ܷ�����ˮ�У���ƿ��������ɫ��dz, �����з�Ӧ��
2NO2
(g)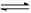 N2O4(g) ��H �еĦ�H 0���>����<����
N2O4(g) ��H �еĦ�H 0���>����<����
��2�� ��ͬѧ��ͼ��װ����ȡNH3��O2�Ļ�����壬��ͼ��װ����֤����ijЩ���ʣ�
��A�м���Ũ��ˮ��B�м���Na2O2���壬C�м����ʯ�ң�D�ڷ��ô�������ʯ�ޣ�������������a�� b��c�� h���Ӹ����� ��
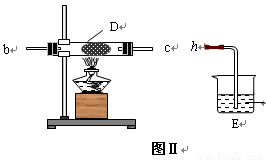
�� ʵ���й۲쵽D���к���ɫ������֣�֤����������____��������ԡ���ԭ�ԡ�����
�� D�з�Ӧ�Ļ�ѧ����ʽΪ________________ _____�� .
�� Ϊ��ֹNO2 ��Ⱦ������Eװ����װ���Լ������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��) ͼ����ʵ�����г������Ʊ����������װ��:
(1) ��ͬѧ��ͼ��װ�á�ͭ��Ũ�����Ʊ����ռ������NO2���壺
�� B�з�Ӧ�Ļ�ѧ����ʽ_____________________________��
�� �ռ�NO2����ķ��� ��
�� ���ռ���NO2����ƿ�ܷ�����ˮ�У���ƿ��������ɫ��dz, �����з�Ӧ��
2NO2(g) N2O4(g) ��H �еĦ�H 0���>����<����
��2�� ��ͬѧ��ͼ��װ����ȡNH3��O2�Ļ�����壬��ͼ��װ����֤����ijЩ���ʣ�
��A�м���Ũ��ˮ��B�м���Na2O2���壬C�м����ʯ�ң�D�ڷ��ô�������ʯ�ޣ�������������a�� b��c�� h���Ӹ����� ��
�� ʵ���й۲쵽D���к���ɫ������֣�֤����������____��������ԡ���ԭ�ԡ�����
�� D�з�Ӧ�Ļ�ѧ����ʽΪ________________ _____�� .
�� Ϊ��ֹNO2 ��Ⱦ������Eװ����װ���Լ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��2012ѧ��㶫ʡ����ѧ���������ܲ⻯ѧ�Ծ� ���ͣ�ʵ����
(16��) ͼ����ʵ�����г������Ʊ����������װ��:
(1) ��ͬѧ��ͼ��װ�á�ͭ��Ũ�����Ʊ����ռ������NO2���壺
�� B�з�Ӧ�Ļ�ѧ����ʽ__________ ___________________��
___________________��
�� �ռ�NO2����ķ��� ��
�� ���ռ���NO2����ƿ�ܷ�����ˮ�У���ƿ��������ɫ��dz,�����з�Ӧ��
2NO2 (g)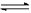 N2O4(g) ��H �еĦ�H 0���>����<����
N2O4(g) ��H �еĦ�H 0���>����<����
��2�� ��ͬѧ��ͼ��װ����ȡNH3��O2�Ļ�����壬��ͼ��װ����֤����ijЩ���ʣ�
��A�м���Ũ��ˮ��B�м���Na2O2���壬C�м����ʯ�ң�D�ڷ��ô�������ʯ�ޣ�������������a�� b��c�� h���Ӹ����� ��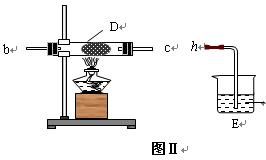
�� ʵ���й۲쵽D���к���ɫ������֣�֤����������____��������ԡ���ԭ�ԡ�����
�� D�з�Ӧ�Ļ�ѧ����ʽΪ________________ _____�� .
��Ϊ��ֹNO2 ��Ⱦ������Eװ����װ���Լ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ����ѧ��ῼ�����ۣ���ѧ���� ���ͣ������
(16��)������һ����Ҫ�Ļ�������ԭ�ϣ���;�㷺��
��1�������ӹ�ҵ�У���ˮ������ʴ��H2O2 ������������ﲻ��Ⱦ�������÷�Ӧ�Ļ�ѧ����ʽΪ2NH3 + 3H2O2= +6H2O
��2����ҵ�г������·�Ӧ�ϳɰ���N2+3H2 2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����N2��H2��Ũ�Ⱦ�Ϊc(N2) = 0.100mol/L, c(H2)
= 0.300mol/L�����з�Ӧʱ, N2��Ũ����ʱ��ı仯��ͼ�١��ڡ���������ʾ��
2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����N2��H2��Ũ�Ⱦ�Ϊc(N2) = 0.100mol/L, c(H2)
= 0.300mol/L�����з�Ӧʱ, N2��Ũ����ʱ��ı仯��ͼ�١��ڡ���������ʾ��

�÷�Ӧƽ�ⳣ������ѧ����ʽΪ ��ʵ���ƽ��ʱH2��ת����Ϊ_____ ��
��3����ͼ��ʾ���ڡ�����װ���и���һ��������ٲ�ͬ����ָ������˵���жϵ����ɡ�
��������_______ ���ɣ� ________
��������_______ ���ɣ� ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��ɽ�и�����ѧģ���Ծ����ţ� ���ͣ������
(16��)������һ����Ҫ�Ļ�������ԭ�ϣ���;�㷺��
�����ӹ�ҵ�У���ˮ������ʴ��H2O2 ������������ﲻ��Ⱦ�������÷�Ӧ�Ļ�ѧ����ʽΪ2NH3+3H2O2= +6H2O
��ҵ�г������·�Ӧ�ϳɰ���N2+3H2 2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����Ũ�Ⱦ�ΪC(N2)=0.100mol/L,
C(H2)=0.300mol/L���з�Ӧʱ, N2��Ũ����ʱ��ı仯����ͼ�١��ڡ���������ʾ��
2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����Ũ�Ⱦ�ΪC(N2)=0.100mol/L,
C(H2)=0.300mol/L���з�Ӧʱ, N2��Ũ����ʱ��ı仯����ͼ�١��ڡ���������ʾ��
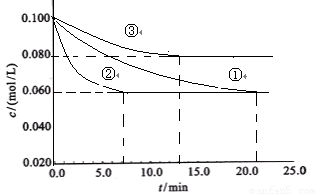
��1���÷�Ӧƽ�ⳣ������ѧ����ʽ ��ʵ���ƽ��ʱH2��ת����Ϊ_______
��2����ͼ��ʾ���ڡ�����װ���и���һ��������ٲ�ͬ����ָ������˵���жϵ����ɡ�
��������_______ ���ɣ� ________
��������_______ ���ɣ� ________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com