
(1)��ͼ������ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B��������
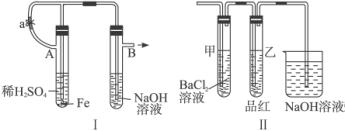
����1���ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������____________________________________________________________________��
B���з�����Ӧ�����ӷ���ʽ��______________________________________________��
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ����ټн�ֹˮ��a��ʵ������B���й۲쵽��������________________________________________________________________��
(2)����װ�õ�ʵ�������ȡA���ڷ�Ӧ������Һ����������С�����ɺ��ٸ������գ��й�װ�ü���������ȥ����������º�ɫ���塣���ֽ�ʱ���������尴ͼ����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�������Һ��ɫ��ɫ���ش�
���û�ѧ����ʽ˵���Թܼײ�����ɫ������ԭ��������˵����____________________��
�ڸ���ʵ������д��ͼ����A������Һ���ɺ��ڸ��������շֽ�ʱ��������������ԭ��Ӧ�Ļ�ѧ����ʽ_____________________________________________________��
�����Ӧ����������_______________________����ԭ����____________________________��
(1)����1���ȳ��ְ�ɫ��״������Ȼ�����Ѹ�ٱ����ɫ������Ϊ���ɫ
Fe2++2OH-====Fe(OH)2�� 4Fe(OH)2+O2+2H2O====4Fe(OH)3
����2����ֹˮ��ʱ����Һ�еĵ��ܿ�������ð�����н����Ӻ��ְ�ɫ��״������һ��ʱ���ڳ�������ɫ
��2����SO3+BaCl2+H2O====BaSO4��+2HCl
��2FeSO4![]() Fe2O3+SO2��+SO3�� FeSO4 FeSO4
Fe2O3+SO2��+SO3�� FeSO4 FeSO4
�����������漰��϶࣬�ȿ���ѧ����ʵ���������ֿ���˼ά�������ԡ������Ժʹ����ԡ������һ������Χ��Fe(OH)2��ʵ�����Ʒ���Ƶġ�����Fe(OH)2���ױ�����Ϊ?Fe(OH)3����Һ���ܽ��O2���ܰ�Fe(OH)2���������н�aʱ��A�Թܱ����һ�������ϵ��������H2ʹA�Թ���ѹǿ�������ɵ�FeSO4��Һѹ��B�У��Ӷ�������Ӧ����?Fe(OH)2���ɡ���NaOH��Һ���ܽ��O2�ɽ�Fe(OH)2Ѹ�����������ת����Fe(OH)3�����ԣ�������ɫ������ʱ��̡ܶ������в���2ʱ��������H2����ͨ��������ͨ�뵽B��NaOH��Һ�У��������ܽ��O2���ߣ��ټн�aʱ��FeSO4��Һѹ��B�У���ʱ��Ӧ��������Fe(OH)2������һ��ʱ���ڲ���ɫ������ڶ�����Χ��FeSO4��������ǿ��ʱ�IJ���������������������ѧ����������ԭ֪ʶ�ƶϳ�FeSO4���·ֽⷴӦ�Ļ�ѧ����ʽ��


 ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���������ص���ѧ����һ��2008�߿���ѧģ����һ ���ͣ�058
��������ʵ��װ�úͲ������ش��й����⣮
(1)��ͼ����ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B��������
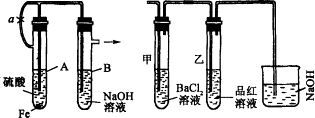
����1���ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽�������ǣ�_____________________________________________
B���з�����Ӧ�����ӷ���ʽ�ǣ�_______________________
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ��������н�ֹˮ��a��ʵ������B���й۲쵽�������ǣ�____________________________��
B���з�����Ӧ�����ӷ���ʽ�ǣ�_________________________
(2)����װ�õ�ʵ�������ȡA���ڷ�Ӧ������Һ����������С�����ɺ��ٸ�������(�й�װ�ü�����������ȥ)��������º�ɫ���壮���ֽ�ʱ���������尴ͼ��ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�������Һ��ɫ��ȥ���ش�
����1���û�ѧ����ʽ˵���Թܼײ�����ɫ������ԭ��(��������˵��)��
����2������ʵ������д��ͼ��A������Һ���ɺ��ڸ������շֽ�ʱ��������������ԭ��Ӧ�Ļ�ѧ����ʽ_____________________�������Ӧ����������________����ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B��������

����1���ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ����B�ܹ۲쵽��������_______________��B�ܷ�����Ӧ�����ӷ���ʽ��____________________________________________________��
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ����ټн�ֹˮ��a��ʵ������B�ܹ۲쵽��������_____________________��B���з�����Ӧ�����ӷ���ʽ��?__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ6-27����ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B���е�����

ͼ6-27
�������ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������_____________��B���з����ķ�Ӧ�����ӷ���ʽ�ǣ�___________________________��
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ����ټн�ֹˮ��a��ʵ������B���й۲쵽��������_________________��B���з����ķ�Ӧ�����ӷ���ʽ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʵ��װ�úͲ������ش��й����⣺
��������ͼ������ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B��������
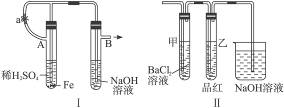
����1���ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������______________
______________________________________________________________________________��
B���з�����Ӧ�����ӷ���ʽ��___________________________________________________��
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ����ټн�ֹˮ��a��ʵ������B���й۲쵽��������_____________________________________________________________________��
��2������װ�õ�ʵ�������ȡA���ڷ�Ӧ������Һ����������С�����ɺ��ٸ������գ��й�װ�ü���������ȥ����������º�ɫ���塣���ֽ�ʱ���������尴ͼ����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�������Һ��ɫ��ɫ���ش�
���û�ѧ����ʽ˵���Թܼײ�����ɫ������ԭ��������˵����____________________��
�ڸ���ʵ������д��ͼ����A������Һ���ɺ��ڸ��������շֽ�ʱ��������������ԭ��Ӧ�Ļ�ѧ����ʽ________________________________________________________________��
�����Ӧ����������_______________________����ԭ����____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com