

| ��һ�� | �ڶ��� | |
| ������ͭ��������g�� | m | m |
| ��ϡ�����������g�� | 50 | 100 |
| ������������Һ��������g�� | 100 | 100 |
| B����Ҫ���� | ����ɫ���� | �� |
| 80 |
| m |
| 171 |
| 100g��8.55% |
| 160 |
| x |
| 171 |
| 100g��8.55% |
| 171 |
| 8.55g |
| 233 |
| x |
| 171 |
| 8.55g |
| 98 |
| y |
| 8g |
| 4g+100g+100g-11.65g-32.35g |
| 171 |
| 8.55g |
| 233 |
| x |


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���̸� | B������ | C��Ӳ�� | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������й���Ѹ������һЩ�������ĵĹ���Ϊ�˶����й��ķ�չ��ƵƵ����������֮�٣��ҹ�Ҳ������ǿ�����Ļ�Ӧ����ͼ����һ����ϰ�У��ҹ����������µ�һ�Ρ������ꡱ���������ˣ����������ѧ֪ʶ�Իش�
�������������й���Ѹ������һЩ�������ĵĹ���Ϊ�˶����й��ķ�չ��ƵƵ����������֮�٣��ҹ�Ҳ������ǿ�����Ļ�Ӧ����ͼ����һ����ϰ�У��ҹ����������µ�һ�Ρ������ꡱ���������ˣ����������ѧ֪ʶ�Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͥʳ������Ҫ�ɷ�Ϊ̼���ƣ����������������Ȼ��ƣ�ij����̽��С���ͬѧΪ�ⶨʳ�������̼���Ƶ�������������Ʋ�����������ʵ�飺��ȡ�����Ʒ11g��ȫ���ܽ���50 gˮ�У������м���ϡ���ᣬ��������������Ϊֹ����ͼ������ϡ�����������������������Ĺ�ϵͼ����������м��㣺
��ͥʳ������Ҫ�ɷ�Ϊ̼���ƣ����������������Ȼ��ƣ�ij����̽��С���ͬѧΪ�ⶨʳ�������̼���Ƶ�������������Ʋ�����������ʵ�飺��ȡ�����Ʒ11g��ȫ���ܽ���50 gˮ�У������м���ϡ���ᣬ��������������Ϊֹ����ͼ������ϡ�����������������������Ĺ�ϵͼ����������м��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
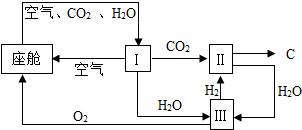 �������Ա�������ڿ������¹�����ͼ��ʾ��
�������Ա�������ڿ������¹�����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com