
6�֣���ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�顣Aװ�ü���ƿ��װ�������ԼΪ1:1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᡣ

��1���رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A�к���Һ��Ϊ��ɫ��B�г���������Һ�档
��ش𣺢���a�����Ƕ�����̼����b�� ��Һ������b��ˮ��������a���е������� ��
��2������K1����״̬������K2��һ��ʱ���ر�K2�����������У��۲쵽D�е������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
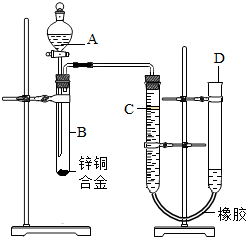 ��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ����������
��2012?��Ӧ�ض�ģ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ����������������֪����������ʱ�ų�һ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
6�֣���ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�顣Aװ�ü���ƿ��װ�������ԼΪ1:1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᡣ

��1���رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A�к���Һ��Ϊ��ɫ��B�г���������Һ�档
��ش𣺢���a�����Ƕ�����̼����b�� ��Һ������b��ˮ��������a���е������� ��
��2������K1����״̬������K2��һ��ʱ���ر�K2�����������У��۲쵽D�е������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���ж������п�һģ��ѧ�Ծ� ���������� ���ͣ������
6�֣���ʦ����ͼ��ʾװ��Ϊͬѧ������һ��ʵ�顣Aװ�ü���ƿ��װ�������ԼΪ1:1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᡣ
��1���رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A�к���Һ��Ϊ��ɫ��B�г���������Һ�档
��ش𣺢���a�����Ƕ�����̼����b�� ��Һ������b��ˮ��������a���е������� ��
��2������K1����״̬������K2��һ��ʱ���ر�K2�����������У��۲쵽D�е������� ��Cװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��Ӧ�ؾ��꼶�����ľ���Ӧ�Բ��Ի�ѧ�Ծ��������棩 ���ͣ�̽����
ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���ͭ������������(��֪����������ʱ�ų�һ��������)

(1)ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���� ��
(2)ʵ����������У���������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú��ٽ��еIJ������У��ټ�¼C��Һ��λ�ã��ڴ�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ����ܼ�������ԡ�
�����ٽ��еIJ�����˳���� (�����)��
(3)��װ�õ������Եļ��鷽���Ǵ�Һ©����������D��ע�뾭���õ� ��ʹU������Һ����ƽ���رշ�Һ©�����������D�ܣ� ��˵��װ�õ������Ժã�
(4)��B�в����������������¼C��Һ��λ��ǰ���轫�¶Ȼָ������º� ��
(5)��ʵ����пͭ�Ͻ������Ϊag����ϡ�����ַ�Ӧ����������ΪV L��Ϊ����Ͻ���ͭ��������������ȱ�ٵ�һ�������� ��
A����Ӧǰ����ϡ�������� B����Ӧǰ����ϡ�������������
C��ʵ��ǰ��Ӧװ���п�������� D��ʵ���������������ܶ�
(6)��aΪ0.4g��VΪ80mL����Ͻ���ͭ������������(��ʵ�������£�H2���ܶ�Ϊ0.09g/L��5��)
(7)ʵ������У���δ��ȴ�Ͷ�ȡ�������������п������������ (�� ��ƫ����ƫС������Ӱ�족)��
(8)��ָ����ʦ��Ϊ����������ʵ��װ�ã���������ܻ�ƫС��ԭ���� ���Ľ������� ��
��������(1) �Ƚ�пͭ�Ͻ���ϡ���н���Ƭ�̣���Ŀ���dz�ȥ����������(2)����ʵ������IJ�����н��3������װ�õ������Եļ��鷽�����н��(5)��������=��������ܶȽ��н��(6)�����֪���������������ݻ�ѧ����ʽ���п�������������ͭ����������(7) δ��ȴ�������ƫ������������ƫ�����п������������ƫ��8��������Ӱ������������������ؽ��н��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com