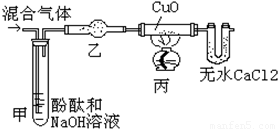
ͼ1��ʵ���ҳ��õ�װ�ã���ش��������⣺
��1��д�������ٵ�����
����©��
����©��
���ռ�ij�����ܲ���Eװ�ã��ɴ��Ʋ��������е�������
�ܶȱȿ���С
�ܶȱȿ���С
��
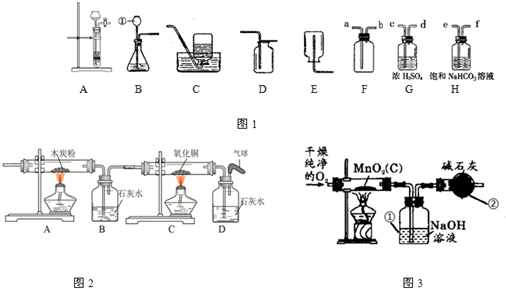
��2��ʵ�����Ʊ����ռ�������װ�������
A����B��C����D��
A����B��C����D��
����װ����ţ�����Ӧ�Ļ�ѧ����ʽΪ
������Fװ���ռ������壬������Ӧ��
a
a
�˽��루�a����b������װ��A��B������������ȡ������̼���Ƚ�������װ�ã�ָ��Aװ�õ�һ��ͻ���ŵ���
������ʱ���Ʒ�Ӧ�ķ�����ֹͣ
������ʱ���Ʒ�Ӧ�ķ�����ֹͣ
��
��3��ʵ�����Ƶõ�CO
2�����г�����HCl��ˮ������Ϊ�˵õ������������CO
2���壬����װ�õĵ���������������˳����
C
C
����ѡ����ĸ��ţ���
A��c��d��e��f B��d��c��e��f C��e��f��c��d D��f��e��d��c
��4��������ͨ����ͼ2��ʾ��װ�ã�ʵ���й۲쵽B�г���ʯ��ˮ����ǣ�C�к�ɫ�����Ϊ��ɫ�������װ��B������һ������
һ����̼�Ͷ�����̼
һ����̼�Ͷ�����̼
��B��������Ӧ�Ļ�ѧ����ʽ��
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
��
��5��ʵ������ȡ����ʱ���ö���������������ij����������Ʒ�к�������̿��Ϊ�ⶨ����Ʒ�ж������̵�����������ij��ȤС����Ƶ�ʵ�鷽���ǣ���һ��������Ʒ��ͨ����﴿����������ʹ����̿�ڼ��������·�Ӧ����CO
2�����з����ⶨ��
����ͼ3��ʾ���ø��﴿������������Ʒ��Ӧ���ⶨ������������������װ�ã���������װ�м�ʯ�ң������ƺ��������ƵĻ�������������
���տ����еĶ�����̼
���տ����еĶ�����̼
��
��Ϊ��֤װ�â��ѽ�CO
2������ȫ������װ�â����֮�����
ʢ�г���ʯ��ˮ��ϴ��ƿ
ʢ�г���ʯ��ˮ��ϴ��ƿ
װ�ý���֤����
���ֳ�ȡ5.0g����������Ʒ����ʵ�飬װ�âٷ�Ӧǰ���������Ϊ1.1g������Ʒ�ж������̵���������Ϊ���٣���д��������̣�