��ʽ̼��ͭ��һ����ɫ�Ĺ��壬���ȷֽ�ɺ�ɫ�������ʡ�������̼��ˮ�����ֺ�ɫ����ijɷ���ʲô�أ��������ѧ�ǽ�������̽����
[����]���ú�ɫ�����������ͭ��Ҳ����������ͭ��̿�Ļ���
[�����ʵ��]
| ʵ�鲽�� |
ʵ������ |
���ۺͻ�ѧ����ʽ |
| ����ͬѧȡ������ɫ���壬�����Թ��У�����������ϡ���ᣬ�ȣ� |
��ɫ������ȫ��ʧ����Һ�� �� �� ɫ�� |
�˺�ɫ����������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ CuO+H2SO4�TCuSO4+H2O CuO+H2SO4�TCuSO4+H2O |
����С����ͬѧ����������ԭ����ͭ��ȡͭ��
��1��Ҫ�Ƶô�����������С��Ӧѡ�õ�ҩƷ��
D
D
A������ϡ���� B��п��Ũ���� C��þ��ϡ����D��п��ϡ����
��2��ʵ�鿪ʼʱ��Ӧ��ͨ��һ���
H2
H2
��Ȼ���ټ��ȣ�����������Ŀ����
��ֹ����ʱ������ը
��ֹ����ʱ������ը
��3��ʵ�������С��ͬѧ�Ի�ԭ��ĺ�ɫ���ʲ�������Ȥ���ú�ɫ����һ���ǵ���ͭ��
�����й����ϻ�֪������������ͭ�ڼ���������������������ͭ��Cu
2O����������ͭҲ�ʺ�ɫ��������ϡ���ᷴӦ��������ͭ��ͭ��ˮ��
Ϊ�ˣ�С��ͬѧ������������ʵ�飬��һ�γ�ȡ4.0g����ͭ����ʵ�飬�õ���3.3g��ɫ���ʣ��ڶ���ͬ����4.0g����ͭ����ʵ�飬�õ���3.2g��ɫ���ʣ�
��С��ͨ��������ʵ�����ݱ��м��������֪t�ڶ���ʵ��õ��ĺ�ɫ����ȫ����ͭ����һ�εõ��ĺ�ɫ���ʳ�ͭ�⣬������������ͭ��С���ķ���������
4gCuO�к��е�ͭԪ������Ϊ3.2g����������ͭ������Ϊ3.2g
4gCuO�к��е�ͭԪ������Ϊ3.2g����������ͭ������Ϊ3.2g
��
��������û�ѧʵ�鷽����֤�����������������ȷ�ԣ�����֤�����ǣ���Ҫд�����������ۣ�
�ֱ�ȡ���ֺ�ɫ�������Թ��У�����ϡ���ᣮ��һ���Թ�����Һ�������ڶ����Թ�����Һ�����������һ�εIJ����к���������ͭ���ڶ��ε���ͭ
�ֱ�ȡ���ֺ�ɫ�������Թ��У�����ϡ���ᣮ��һ���Թ�����Һ�������ڶ����Թ�����Һ�����������һ�εIJ����к���������ͭ���ڶ��ε���ͭ
�۴�����ʵ���У�С��������½��ۣ���������ԭ����ͭʵ���У�����ԭ����֣�һ��õ���ɫ������ͭ����д��������ԭ����ͭ����������ͭ�Ļ�ѧ����ʽ
��

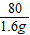 =
=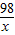
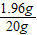 ×100%=9.8%
×100%=9.8% 

 ��У����ϵ�д�
��У����ϵ�д�

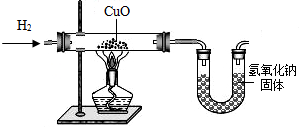
 ijѧ������ͼ��ʾװ���Ʊ������������Ƶõ�������ԭ����ͭ��װ���б�Ҫ������̨�����С������豸����ͼ�о�����ȥ��������д���пհף�
ijѧ������ͼ��ʾװ���Ʊ������������Ƶõ�������ԭ����ͭ��װ���б�Ҫ������̨�����С������豸����ͼ�о�����ȥ��������д���пհף�