
| �����ƻ���--�����״� ��Ҫ�ɷ֣�̼����泥�NH4HCO3�� ��������ȫ��֮�ף�24% ��������50kg/�� �����桱�����������ι�˾��Ʒ |
 ×100%�����з������
×100%�����з������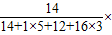 100%��17.72%��
100%��17.72%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��������̼����泥�NH4HCO3������ҵ������һ�����ͻ����森��ͼ��ʾ�����������Ϣ���㣺��N-14H-1C-12O-18��
ij��������̼����泥�NH4HCO3������ҵ������һ�����ͻ����森��ͼ��ʾ�����������Ϣ���㣺��N-14H-1C-12O-18���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �����ƻ���--�����״� ��Ҫ�ɷ֣�̼����泥�NH4HCO3�� ��������ȫ��֮�ף�24% ��������50kg�� �����桱�����������ι�˾��Ʒ��1��̼����泥�NH4HCO3���е����⡢����̼����Ԫ�ص������� ��2��̼����泥�NH4HCO3���е�Ԫ�ص���������Ϊ ��3��ͨ�������жϸù������ ��4��ÿ���û����е�Ԫ�ص����� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ij��������̼����淋���ҵ��������һ�����棺  ��������ѧ��֪ʶ����������һ���������Ƿ���ʵ�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ˮ������֮Դ������֮����������������Ȼ��Դ��  һ����1���������������ˮ����Ⱦ���У�����ţ� AD AD ��A����ҵ��ˮֱ���ŷ� B����ҵ�����������ŷ� C����ֹʹ�ú���ϴ�·� D������ʹ�û��ʡ�ũҩ ��2����ͼ1��ˮͨ��ֽ��ʾ��ͼ����ʵ������У��Թ�a�в����������� ���� ���� ����3��Ϊ�˳�ȥˮ�е����ʣ�ijͬѧ��������ͼ2��ʾ�ļ���ˮ�������л���̿����Ҫ������ ����ɫ�غ���ζ ����ɫ�غ���ζ ������ˮ��Ӳˮ������ˮ�����ǿ��õ�����������ˮ ����ˮ �������У�������� ��� �ķ�������ˮ��Ӳ�ȣ������ڻ�ѧ��Ӧ�У�ˮ�ȿɳ䵱��Ӧ��Ҳ�ɳ䵱�������ͼ3��ʾ���ֻ�������ת����ϵ��dz��ɫ��ĩ����A���ȷֽ�Ϊ������B��BΪ��ɫ���壩��������C��CΪ��ɫ���壩��ˮ��E��D�ֱ�������Ԫ����ɣ�����D�ֽܷ�ΪC��H2O��EΪ��ɫ���ܽ���ˮ�Ĺ��壮����ͼʾ��ϵ���ش��������⣺ ��д���й����ʵĻ�ѧʽ��C CO2 CO2 ��DH2CO3 H2CO3 ��ECaCO3 CaCO3 ����д����Ӧb�����ֱ���ʽ ˮ+������̼��̼�� ˮ+������̼��̼�� ����������������ˮ����������ķ����Ĵ��˺�������ͬ�Ӷ�ˮ��Դ����״���е�һ�������ݣ�
��С��ͬѧͨ�������жϸù������ٹ�棬�������� ����к�����Ϊ24%������̼������еĺ�����17.7%�� ����к�����Ϊ24%������̼������еĺ�����17.7%�� ���������ල���ž�����ⷢ�ָû��ʵĺ�����Ϊ17.5%��������NH4HCO3����������Ϊ 98.87% 98.87% ���� 25.0 25.0 ǧ�˵�����泥�NH4NO3����������ʵĺ�������ȣ��鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ij��������̼����泥�NH4HCO3������ҵ����һ������������Χǽ�����˴��ͻ����棬��������������̼����狀���20%��Ϊȫ����ߣ���ͨ�������жϣ��ù���Ƿ�������ٹ�森 �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |