
����֮�������ϵ���ҳ���ϵ�����ֹ���������ѧ�û�ѧ��һ�ֻ���������
��1����������±��е�ʾ�����ҳ�CO2��CH4��C2H2����Ȳ����C2H4����ϩ������������֮����ڵ������������ɲ�����
���� ����
ʾ������״���£��ܶ��ɴ�С CO2��C2H4��C2H2��CH4
��2��ij������CH4��C2H2��C2H4�е�һ�ֻ�����ɣ�ȡ��������Ʒ����������ȫȼ�գ�������ɵĶ�����̼��ˮ��������Ϊ22��9�����жԸ���������ж���ȷ����������
A����������һ������C2H4
B����������һ��û��CH4
C���������п��ܺ���C2H2
D�������岻����ͬʱ����CH4��C2H2��C2H4��
�����㡿�йػ�ѧʽ�ļ�����ƶϣ������غ㶨�ɼ���Ӧ�ã�
��ר�⡿��ѧʽ�ļ��㣻��ѧ����������غ㶨�ɣ�
�����������ݻ�ѧʽ��ʾ���壬�ɴ���Է��������Ĵ�С��һ������������ԭ�Ӹ����Ķ��ٵ���Ѱ�ҹ��ɣ����ݶ�����̼��ˮ��������Ϊ22��9���ɼ����̼��������Ԫ�ص������ȣ��ݴ˽��
����𡿽⣺��1��CO2��CH4��C2H2����Ȳ����C2H4����ϩ��������������Է��������ֱ��ǣ�44��16��26��28��������Է���������С�����˳����CH4��C2H2��C2H4��CO2��
CO2��CH4��C2H2����Ȳ����C2H4����ϩ������������һ������������ԭ�Ӹ����ֱ���3��5��4��6������һ�����ӷ���������ԭ�Ӹ������ٵ����˳����CO2��C2H2��CH4��C2H4��
�ʴ�Ϊ��
���� ����˳��
��״���£���Է���������С���� CH4��C2H2��C2H4��CO2
ÿ�������к���ԭ��������С���� CO2��C2H2��CH4��C2H4
��2�����ɵĶ�����̼��ˮ��������Ϊ22��9����̼Ԫ�غ���Ԫ�ص�������Ϊ����22��
 ������9��
������9��
 ��=6��1����̼��ԭ�ӵĸ�����Ϊ��
��=6��1����̼��ԭ�ӵĸ�����Ϊ��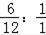
 =1��2��
=1��2��
A�����Ǻ�һ��CH4��һ��C2H2�����ܴﵽ̼��ԭ�Ӹ�������1��2���������в�һ������C2H4������
B�����Ǻ�һ��CH4��һ��C2H2�����ܴﵽ̼��ԭ�Ӹ�������1��2������
C���������п��ܺ���C2H2����ȷ��
D�����������ͬʱ����CH4��C2H2��C2H4������
����C��
����������Ҫ��������Է��������ļ���ͷ��ӵĹ��ɣ����ݻ�ѧʽ�����йص��ƶ�ʱ��Ҫ��������غ㶨���Լ���ѧʽ���йؼ�����У�
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����еĻ�ѧʽ��������ѧ���ܱ�ʾͬһ���ʵ���( )
A��Ca��OH��2 ��ʯ�� �������� B��KOH �ռ� ��������
C��CaO ��ʯ�� ������ D��NaCl ʳ�� �Ȼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ʻ֤�����ʹ�ù涨�������������123�ţ�2013��1��1����ʩ�У����ݡ���·��ͨ��ȫ�����ľ�����ȡ����ԭ���ƺ��ʻ��������12�ֵ����ݣ��涨���ƺ��ʻ��������12�֣�����ʻԱ�Ƿ�ƺ�ݳ����ƾ�������еķ�Ӧԭ��Ϊ��
C2H5OH+4X������ɫ��+6H2SO4�T2Cr2��SO4��3����ɫ��+2CO2��+9H2O��������X�Ļ�ѧʽΪ��������
A��CrO3 B��Cr2O3 C��Cr2S3 D��CrSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��������п��ھƾ��ƻ�����ֱ�Ӽ��ȵ��ǣ�������
A���Թ� B���ձ� C����ƿ D����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й���֬��������ȷ���ǣ�������
A��������֬������̼���⡢������Ԫ����ɵ�
B�������Dz���ת��Ϊ�����ڵ�֬��
C�����͡�ú�͡������͡������Ͷ�������֬
D��ϴ����ϴȥͷ���ϵ���֬�������黯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У�û�з�����ѧ�仯���� ��������
A�����ڷ��õij���ʯ��ˮ���а�ɫ��������
B�����ڷ��õ��Ȼ��Ʊ�����Һ���а�ɫ��������
C��Ũ���ὦ��ľ���ϣ�ľ�ı��
D�����������ڵ��µ������ϲ������ɫ�ߵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�̼��̼���������˵��������� ��������
A�����ʯ��ʯī���������ʲ�ͬ������Ϊ���ǵ�̼ԭ�����з�ʽ��ͬ
B��CO��CO2�����Ԫ����ͬ���������ǵĻ�ѧ���ʲ�ͬ
C��ľ̿�ͻ���̿������������
D��CO2ͨ����ɫʯ����Һ����Һ��죬���Ⱥ���Һ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ǻ�ѧѧϰ�г��õ�˼ά����������������ȷ���ǣ�������
A�������ﶼ���ɲ�ͬ��Ԫ����ɵģ���ͬԪ����ɵĴ�����һ���ǻ�����
B�����ж����⡢��Ԫ�أ����Ժ��⡢��Ԫ�صĻ����ﶼ�Ǽ�
C���кͷ�Ӧ���κ�ˮ���ɣ������κ�ˮ�ķ�Ӧ�������кͷ�Ӧ
D����������һ��������Ԫ�أ�����һ��Ԫ��һ���ǽ���Ԫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com