

 [ʵ�����]������ȷ��
[ʵ�����]������ȷ��| ʵ�鲽�� | ʵ������ | ʵ����� | |
| �٣�19�� | �ڼ��쵼�ܴ���ȼ���壬 �ڼ��쵼�ܴ���ȼ���壬�ڻ����Ϸ���һֻ������ձ� �ڼ��쵼�ܴ���ȼ���壬�ڻ����Ϸ���һֻ������ձ� �� |
�ձ��ڱ���ˮ�� �ձ��ڱ���ˮ�� |
ԭ��������������� ԭ��������������� �� |
| �ڣ�20�� | �������ձ���Ѹ�ٵ�����������ʯ��ˮ�������ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ����� �������ձ���Ѹ�ٵ�����������ʯ��ˮ�������ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ����� �� |
����ʯ��ˮ����ǣ���������ɫ���� ����ʯ��ˮ����ǣ���������ɫ���� |
ԭ�����������һ����̼ ԭ�����������һ����̼ |
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| �٣�19�� | �ڼ��쵼�ܴ���ȼ���壬�ڻ����Ϸ���һֻ������ձ��� | �ձ��ڱ���ˮ�� | ԭ����������������� |
| �ڣ�20�� | �������ձ���Ѹ�ٵ�����������ʯ��ˮ�������ڻ����Ϸ���һֻ�ڱ�Ϳ�г���ʯ��ˮ���ձ������� | ����ʯ��ˮ����ǣ���������ɫ���� | ԭ�����������һ����̼ |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
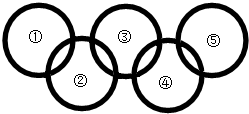 ��2012?������һģ����ͼ������������Բ���������һ�������¿��Է�����Ӧ����������ѡ���У����ϸ�ͼҪ����ǣ������� ��2012?������һģ����ͼ������������Բ���������һ�������¿��Է�����Ӧ����������ѡ���У����ϸ�ͼҪ����ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 9 |
| 25 |
| 9 |
| 25 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com