
���϶�һ�š�����̽��������2007��10��24�ճɹ����䣬ʵ�����й�����ķ����Σ����δ˴η�������������ǡ��������żס����ػ��������ȼ����Һ̬������ȼ����Һ̬����ȼ��ʱ��������ƶ��������϶�һ�š�������̫�գ�
��1����ȼ��ȼ����Ҫ��������������������
��2������ȼ�յĻ�ѧ����ʽ��������
��3��������Ա������ֱ�����ڿ�����ȼ�������������Ǹɷۣ�����Ҫ�ɷ���̼�����ƣ�̼��
�����ڼ���״̬�·����ֽⷴӦ��������ˮ��������̼��̼���ƣ�Na��ɫ�к�ը��ɳ�ش�ʱʹ��ƶ�˵����شٹ���2CO3������д��̼�����ƣ�NaHCO3�����ȷֽ�Ļ�ѧ����ʽ��������
�����㡿ȼ����ȼ�յ���������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�������Ļ�ѧ������ȼ��ʵ�飮
��ר�⡿��ѧ����Դ��
����������1������ȼ�յ�������ͬʱ���������������ٿ�ȼ���������������۴ﵽȼ�����������¶ȼ��Ż�㣩���з�����ɣ�
��2����������ȼ������ˮ��ϻ�ѧ����ʽ����д������
��3�����ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
����𡿽⣺��1������ȼ�յ�������֪������ȼ�ձ�����п�ȼ�ԣ���ȼ�������������������Ӵ����¶ȱ���ﵽȼ�����������¶ȼ��Ż�㣬����������ȱһ���ɣ��ʴ�Ϊ������������������Ӵ����¶ȴﵽ��ȼ����Ż�㣮
��2������ȼ����������������Ӧ����ˮ�������ǵ�ȼ�����Է�Ӧ����ʽΪ��2H2+O2
 2H2O���ʴ�Ϊ��2H2+O2
2H2O���ʴ�Ϊ��2H2+O2
 2H2O��
2H2O��
��3��̼�������ڼ���״̬�·����ֽⷴӦ��������ˮ��������̼��̼���ƣ�Na2CO3������Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3
 Na2CO3+H2O+CO2����
Na2CO3+H2O+CO2����
���2NaHCO3
 Na2CO3+H2O+CO2����
Na2CO3+H2O+CO2����
����������Դ����Ϣ�����ϡ������ǵ��������Ĵ���Ҫ���⣬����ԴΣ���������صĽ��죬����������ȵ㣬Ҳ�ǻ�ѧ������ȵ㣬�ر������ܵķ��ࡢ�ŵ㡢��ȡ�������������ۡ��������Ѻ�ǰ���ȣ�
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���ǣ�������
A����100gKCl������Һ���ɵõ�25g���壬��KCl�ڸ��¶��µ��ܽ��Ϊ25g
B�����ʵ��ܽ����ͨ��������������ı仯
C��������Һʱ�������������������ʵ��ܽ��
D��60��ʱ����ص��ܽ��Ϊ110g������Һ���������ܼ���������Ϊ11��21
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
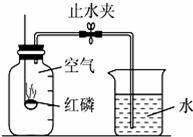
ijͬѧ����˲ⶨ����������������ʵ�飬ʵ��װ����ͼ����ͬѧ��ʵ�鲽�����£�
�ٽ�ͼ�еļ���ƿ�ݻ���Ϊ5�ȷݣ������ñ�ǣ�
���ھƾ����ϵ�ȼ���ף����������뼯��ƿ�ڣ�������Ƥ����
�۳�ַ�Ӧ������ƿ��ȴ�����£����ɼУ�
��ش��������⣺
��1����ʵ���к������Թ�����Ŀ��������
��2������ڼ���ƿ�е�����������
�ɲ�����д��ɼк�۲쵽������ɵó��������������������ԼΪ�� ������ʵ�����������ƿ��ˮλ��������Ԥ��λ�ã���ʵ��ʧ�ܵ�ԭ�������������д��һ�㣩��
������ʵ�����������ƿ��ˮλ��������Ԥ��λ�ã���ʵ��ʧ�ܵ�ԭ�������������д��һ�㣩��
��3��д������ȼ�յ����ֱ���ʽ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ͣ�C3H5N3O9�������������Ľ�ʹ����Ϊ���������ڱ�������������A���壺4C3H5N3O9+5O2�T12A+12CO2+10H2O����A�Ļ�ѧʽΪ��������
A��NO B��NO2 C��N2 D��N2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ס������ֽ���Ƭ�ֱ��������ͭ��Һ�У��ױ�����������ͭ����û�з�����Ӧ���ݴ��жϣ����ֽ����Ļ��˳���ǣ�������
A���ף�ͭ���� B��ͭ���ף��� C���ң�ͭ���� D���ף��ң�ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҳ���п��ϡ���ᷴӦ����ȡ������ijͬѧȡ6.5g��пƬ������50g���ʵ���������һ����ϡ���ᣬǡ����ȫ��Ӧ���õ�56.3g����п��Һ�����㣺
��1������������������
��2������ϡ�������ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϡ���ᡢ�������ơ���ˮ����PHֵ�ɴ�С��˳��Ϊ��������
A��H2SO4��H2O��NaOH B��NaOH��H2O��H2SO4
C��H2SO4��NaOH��H2O D��NaOH��H2SO4��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һЩ��������ʵ���о�����Ҫ����գ�


��1��ģ�ҵ������װ��ͼ��ͼ������Ӳ�ʲ������ڼ���������������ĩ����ͨ��һ����̼���ų�װ���ڿ��������þƾ���Ƽ���A��ҩƷ����Ӳ�ʲ������ڷ�����Ӧ�Ļ�ѧ����ʽΪ������
���Թ�B�з�����Ӧ�Ļ�ѧ����ʽΪ���������۸�װ�õ���Ҫȱ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йش�����˵���д�����ǣ�������
A���������Ƕ�������
B�������ܸı��������ʵĻ�ѧ��Ӧ�ٶ�
C���ڻ�ѧ��Ӧǰ�����������û�иı�
D���ڻ�ѧ��Ӧǰ������Ļ�ѧ����û�иı�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com