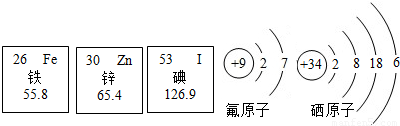
��2013?�����һģ����1�������б������Ԫ����������п��������ȣ���Ȼ�����٣����Խ���������Ҫ�������ṩ������Ԫ�ص������Ϣ��������������ش��������⣺
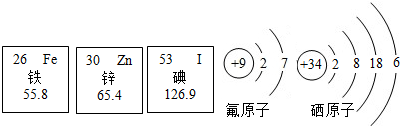
��������Ԫ�������ڷǽ���Ԫ�ص���
3
3
�֣�
����ԭ�Ӻ��������Ϊ
26
26
��
����ԭ���ڻ�ѧ��Ӧ�����õ�2�����ӣ��γ�
��
��
��ѡ����������������ӣ�
��2��������ijͬѧ����ѧ���ʵķ�����ɣ�����ϸ�������ش��������⣺
������
| | ���ʣ�Al��He��N2 | ������ | | A��MgO��CO2��H2O2 | | B��NCl��H2SO4��NH4NO3 | | C��NaOH��Ca(OH)2Fe(OH)3 | | D��NaCO3��NaHCO3��KMnO4 |
| |
| | | | |
| |
���C���������������
��
��
��
���ڷ������оٵ������У���һ�����ʹ����������������
NH4NO3
NH4NO3
��
��A�������е�MgO�е������ӷ���Ϊ
Mg2+
Mg2+
��
��D������KMnO
4��Mn�Ļ��ϼ�Ϊ
+7
+7
��
���ڷ������оٵ������У�������������������������Һ��Ӧ������ʽΪ2Al+2NaOH+2H
2O=2NaAlO
2+3X������X�Ļ�ѧʽΪ
H2
H2
��
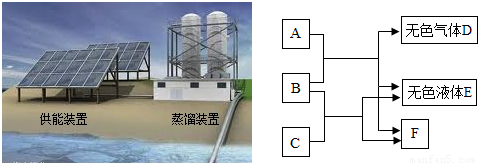
��3���ҹ��Ϻ���������������ɢ���ŷ�Χ����ɺ����Ⱥ���̺��о����Ŀ����Դ����ҵ��Դ�ȣ��С��ڶ�����˹�塱֮�ƣ�
���Ϻ����������ڶ࣬�����ḻ���������ṩ���л�Ӫ������ҪΪ
������
������
��
����ͼΪ��ˮ����װ�ã����õ���Դ��
̫����
̫����
����õ�������װ������װ��ˮ����
B
B
������ĸ��ţ���
A������ B�������� C�������
�۴Ӻ�ˮ����ȡʳ�Σ����õķ�����
�����ᾧ
�����ᾧ
��
���Ϻ��ѳ�Ϊ�ҹ����ĺ��ϻ�ʯȼ���������أ�д����֪����һ�ֻ�ʯȼ��
ú����ʯ�ͣ�����Ȼ��
ú����ʯ�ͣ�����Ȼ��
��
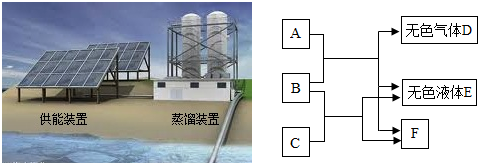
��4����ͼ��ʾ��A��B��C��D��E��F���dz��л�ѧ�������ʣ�A��B�������Ǹ��ֽⷴӦ��B��C���������кͷ�Ӧ��F����������������ز����ٵ�һ���Σ����ͼ����Ϣ���ش��й����⣺
��A�Ļ�ѧʽΪ
Na2CO3��NaHCO3
Na2CO3��NaHCO3
��B�Ļ�ѧʽΪ
HCl
HCl
��F�Ļ�ѧʽΪ
NaCl
NaCl
��
��C��D��Ӧ�Ļ�ѧ����ʽΪ
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
��