
������θ�ᣨ��Ҫ�ɷ������ᣩ���࣬������ܶ�θ������ͼΪij�����װ��ǩ�ϵIJ������֣���ش��������⡣

��1����ҩ��������̷�����ԭ���� ��
��2��ijͬѧΪ�ⶨ��ҩ���������������������������¼����������ȡһƬҩƬ��ҩƬ����Ϊ0.5g����������20mL����ˮ��Ȼ������������Ϊ5%���ܶ�Ϊ1.02g/mL��������з�Ӧ�����������ɷֲ������ᷴӦ����ǡ����ȫ��Ӧʱ����������������Ϊ6.0mL��ͨ�����㣺���жϸ�ҩƬ�����������ĺ����Ƿ�ﵽ��ע��
��ȷ����ҩƬ��������������������Ϊ���٣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ֢ ��Ӧ֢ ������ҩ��������θ������Ҫ�ɷ� ��Ҫ�ɷ� ÿƬ������������250mgע������ ע������ ������̷���1��ҩ��������̷�����ԭ����ҩ���θ���ֽӴ���Ѹ�ٷ���ҩЧ ҩ���θ���ֽӴ���Ѹ�ٷ���ҩЧ ����2��ͬѧΪ�ⶨ��ҩ���������������������������¼����������ȡһƬҩƬ��ҩƬ����Ϊ0.5g����������20mL����ˮ��Ȼ������������Ϊ5%���ܶ�Ϊ1.02g/mL��������з�Ӧ�����������ɷֲ������ᷴӦ����ǡ����ȫ��Ӧʱ����������������Ϊ0.6mL��ͨ�����㣺���жϸ�ҩƬ�����������ĺ����Ƿ�ﵽ��ע����ȷ����ҩƬ��������������������Ϊ���٣� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 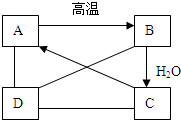 ��A��B��C��D�������ʣ���ͼ��ʾ��A��B��C��һ���¿��Է���ת������C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D����������θ�����Ҫ�ɷ֣� ��A��B��C��D�������ʣ���ͼ��ʾ��A��B��C��һ���¿��Է���ת������C��Һ��ͨ��CO2����Һ����ǣ����ɰ�ɫ����A��D��A��B��C���ܷ�����Ӧ��D��C�����кͷ�Ӧ��D��A��Ӧ��CO2���������D����������θ�����Ҫ�ɷ֣���1���������֪�����ƶ��������ʵĻ�ѧʽ�� A CaCO3 CaCO3 �� BCaO CaO ��CCa��OH��2 Ca��OH��2 �� DHCl HCl ����2����д�����з�Ӧ�Ļ�ѧ����ʽ�� C��Һ��ͨ��CO2�ķ�Ӧ Ca��OH��2+CO2=CaCO3��+H2O Ca��OH��2+CO2=CaCO3��+H2O ��D��A��Ӧ CaCO3+2HCl=CaCl2+H2O+CO2�� CaCO3+2HCl=CaCl2+H2O+CO2�� ��D��C��Ӧ 2HCl+Ca��OH��2=2H2O+CaCl2 2HCl+Ca��OH��2=2H2O+CaCl2 ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ������θ�����Ҫ�ɷ������ᣬθ�������������岻�������֢״������θ�����IJ��˳���ҩ�����ƣ��������ʴ������Ͻ�������������θ���ҩ����ǣ������� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 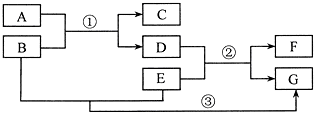 A��G��ʾ���л�ѧ���������ʣ�����֮���ת����ϵ��ͼ��ʾ����������������ȥ��������AΪ���������E������Ŀǰ�����������ߵĽ�����FΪ�Ϻ�ɫ�ĵ��ʣ�B��������θ�����Ҫ�ɷ֣���ѧ��Ӧ�ں͢۵Ļ�����Ӧ������ͬ�� A��G��ʾ���л�ѧ���������ʣ�����֮���ת����ϵ��ͼ��ʾ����������������ȥ��������AΪ���������E������Ŀǰ�����������ߵĽ�����FΪ�Ϻ�ɫ�ĵ��ʣ�B��������θ�����Ҫ�ɷ֣���ѧ��Ӧ�ں͢۵Ļ�����Ӧ������ͬ���밴Ҫ����գ� ��1��д���������ʵĻ�ѧʽ�� A CuO CuO ��BHCl HCl ��EFe Fe ��FCu Cu ��GFeCl2 FeCl2 ����2��д����Ӧ�ٵ�һ����ѧ����ʽ CuO+2HCl=H2O+CuCl2 CuO+2HCl=H2O+CuCl2 ����3��д����Ӧ�ڵ�һ����ѧ����ʽ Fe+CuCl2=FeCl2+Cu Fe+CuCl2=FeCl2+Cu ����4����Ӧ�۵Ļ�����Ӧ������ �û���Ӧ �û���Ӧ ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ���A������B ��ʯ�ң�C�����ʣ�D�ɱ���E���ᣬFʯ��ʯ��ѡ�������������ʣ������������� ��1��������θ�����Ҫ�ɷ��� ��2�������к��������� ��3���������˹�������� ��4��ũҵ���к������������� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |