
����Ŀ����������Ħ����������̼��ƿ����ÿ�ʯ![]() ���Ʊ���ij��ѧ��ȤС�������
���Ʊ���ij��ѧ��ȤС�������![]() ��ת�����̣���ͼ��ʾ��
��ת�����̣���ͼ��ʾ��
���������̣�
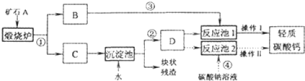
��������ϣ�
![]() ��������̼����ͨ������������Һ�������·�Ӧ��
��������̼����ͨ������������Һ�������·�Ӧ��
![]() ��
��![]() ��
��
![]() ��̼���������ˮ�����ֽ⣺
��̼���������ˮ�����ֽ⣺![]() ��
��
![]() ����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ���
����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ���
���������ۣ�
![]() С�����������̢١��ڡ��ܺͲ���
С�����������̢١��ڡ��ܺͲ���![]() ����ƣ���д����Ӧ�ٺܵ͢Ļ�ѧ����ʽ��
����ƣ���д����Ӧ�ٺܵ͢Ļ�ѧ����ʽ��
��________����________��
����![]() ���������________��ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���Ʒ_____��
���������________��ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���Ʒ_____��
![]() С����Ϊ���̢١��ڡ��ۺͲ���
С����Ϊ���̢١��ڡ��ۺͲ���![]() ��С�������Ÿ��ã������ǣ�________��
��С�������Ÿ��ã������ǣ�________��
![]() ������̼���ʱ��
������̼���ʱ��![]() Ϊ________��ѡ�����Һ������Һ�����������ǣ�________��
Ϊ________��ѡ�����Һ������Һ�����������ǣ�________��
![]() С����Ϊ�õ��IJ�Ʒ�п��ܺ�������
С����Ϊ�õ��IJ�Ʒ�п��ܺ�������![]() �����Բ���
�����Բ���![]() �������衢________���ˡ���ɵȹ�������߲��ʣ�
�������衢________���ˡ���ɵȹ�������߲��ʣ�
����Ʒ�����ⶨ��![]() �����IJⶨ��ȡ
�����IJⶨ��ȡ![]() ��Ʒ���гɷ�״��ͼ����ʵ�飮
��Ʒ���гɷ�״��ͼ����ʵ�飮

![]() ʵ�鲽�裺
ʵ�鲽�裺
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�![]() ����
����![]() ������ͨ��һ��ʱ�������
������ͨ��һ��ʱ�������
�۳���![]() ���������ܹرյ��ɼ�
���������ܹرյ��ɼ�![]() �������μ�ϡ������������ֱ��
�������μ�ϡ������������ֱ��![]() ��������ð����
����������
�ݴ��ɼ�![]() ���ٴλ���ͨһ��ʱ�����������
���ٴλ���ͨһ��ʱ�����������![]() ����������ǰ������������Ϊ
����������ǰ������������Ϊ![]() ��
��
![]() ����̽��
����̽��
�ٲ�Ʒ�гɷ۵�Ŀ��________��
��![]() װ�õ�������________��
װ�õ�������________��![]() װ�õ�������________��
װ�õ�������________��
����û��![]() װ�ã���ⶨ��
װ�ã���ⶨ��![]() ������������________���ƫ����ƫС���������䡱����
������������________���ƫ����ƫС���������䡱����
����![]() ����������ǰ������������Ϊ
����������ǰ������������Ϊ![]() ������ò�Ʒ��
������ò�Ʒ��![]() ����������Ϊ________
����������Ϊ________![]() ��
��
���ܽᷴ˼��
ijͬѧ�������ʵ�鷽���ⶨ�����и�Ԫ�ص�������������һ���������м������ϡ���ᣬ�ⶨ����![]() ���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ��������________��
���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ��������________��
���𰸡�CaCO3![]() CaO+CO2��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����NaOH������̼�õ�������ã���Լԭ������Һ����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�������Ӵ��������ַ�Ӧ����ȥ�����еĶ�����̼����ֹ�����еĶ�����̼����
CaO+CO2��Ca��OH��2+Na2CO3=CaCO3��+2NaOH����NaOH������̼�õ�������ã���Լԭ������Һ����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�������Ӵ��������ַ�Ӧ����ȥ�����еĶ�����̼����ֹ�����еĶ�����̼����![]() װ�ã�ƫ��
װ�ã�ƫ��![]() ��������̼������ˮ
��������̼������ˮ
��������
��1��̼����ֽܷ����������ƣ�����������ˮ��Ӧ�����������ƣ������������������̼��̼���Ʒ�Ӧ����̼��ƣ����ɵ�̼��Ʋ�����ˮ������ͨ�����˵ķ�������Һ�з����������2���������ʵ�ѭ�����÷�������3�������������Ƶ��ܽ��Է�������4������������Ϣ������̼����������̼������Լ�̼������ȷֽ�����ʷ�������6����������ͼ��ʵ��ԭ����Ҫ�ⶨ̼��Ƶ������������ɲⶨ���ɵĶ�����̼��������������Fװ�����ն�����̼��������֮ǰ���ȥ������̼�е�ˮ�ּ������еĶ�����̼�����Ҫ���ó�ȥ������̼�Ŀ��������ɵĶ�����̼ȫ���ŵ�Fװ���������ݶ�����̼���ܽ��Խ����
��1����̼��Ƹ��·ֽ����������ƺͶ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3![]() CaO+CO2������������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����ɵ�̼��Ʋ�����ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+Na2CO3=CaCO3��+2NaOH�������������ˡ�ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���ƷNaOH����2�����̢١��ڡ��۵Ģ��ǽ�̼��Ʒֽ����ɵĶ�����̼�������ã���Լ��ԭ������3��������̼���ʱ��DΪ����Һ�������ǣ�����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�����4����������Ϣ������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+H2O+CO2=Ca��HCO3��2��̼���������ˮ�����ֽ⣺Ca��HCO3��2�TCaCO3��+H2O+CO2����֪��̼�����ת����̼��ƿ��Բ����ȵķ�������6��������Ӵ��������ַ�Ӧ���ɽ���Ʒ�гɷ�ĩ���ڸ�������ͼװ�÷�����Ҫͨ���ⶨ���ɵĶ�����̼����������̼��Ƶĺ��������轫���ɵĶ�����̼�ó�ȥ������̼�Ŀ���ȫ���ų������Bװ�õ������dz�ȥ�����еĶ�����̼�� Gװ�õ������Ƿ�ֹ�����еĶ�����̼����Fװ�ã�����û��Eװ�ã����ɵĶ�����̼�����к���ˮ�ֶ�ʹ������̼������������ⶨ��CaCO3������������ƫ��������F����������ǰ������������Ϊ8.7g�������ɵĶ�����̼������Ϊ8.7g��
CaO+CO2������������������̼���Ʒ������ֽⷴӦ����̼��ƺ��������ƣ����ɵ�̼��Ʋ�����ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+Na2CO3=CaCO3��+2NaOH�������������ˡ�ϴ�ӡ���ɵȹ��������У�ͨ����Ӧ�ܿɻ��ո���ƷNaOH����2�����̢١��ڡ��۵Ģ��ǽ�̼��Ʒֽ����ɵĶ�����̼�������ã���Լ��ԭ������3��������̼���ʱ��DΪ����Һ�������ǣ�����ʯ��ˮ����������Ũ��̫С������Ч�ʺܵ�����4����������Ϣ������̼����ͨ������������Һ�������·�Ӧ��CO2+Ca��OH��2=CaCO3��+H2O��CaCO3+H2O+CO2=Ca��HCO3��2��̼���������ˮ�����ֽ⣺Ca��HCO3��2�TCaCO3��+H2O+CO2����֪��̼�����ת����̼��ƿ��Բ����ȵķ�������6��������Ӵ��������ַ�Ӧ���ɽ���Ʒ�гɷ�ĩ���ڸ�������ͼװ�÷�����Ҫͨ���ⶨ���ɵĶ�����̼����������̼��Ƶĺ��������轫���ɵĶ�����̼�ó�ȥ������̼�Ŀ���ȫ���ų������Bװ�õ������dz�ȥ�����еĶ�����̼�� Gװ�õ������Ƿ�ֹ�����еĶ�����̼����Fװ�ã�����û��Eװ�ã����ɵĶ�����̼�����к���ˮ�ֶ�ʹ������̼������������ⶨ��CaCO3������������ƫ��������F����������ǰ������������Ϊ8.7g�������ɵĶ�����̼������Ϊ8.7g��
��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 8.7g![]()
x=19.77g
��̼��Ƶ���������Ϊ��![]() ��100%=98.86%
��100%=98.86%
���ܽᷴ˼�������ǣ�������̼������ˮ������ʹ��õĶ�����̼���ƫС��


 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�������Һ������ʵ�飮
����![]() ��������Ϊ
��������Ϊ![]() ������������Һ��
������������Һ��
��1�����㣺��Ҫ�������ƹ��������Ϊ________![]() ��ˮ�����Ϊ________
��ˮ�����Ϊ________![]() ��ˮ���ܶȽ��ƿ���
��ˮ���ܶȽ��ƿ���![]() ����
����
��2������������������ƽƽ���________����������ƽ�����̣�������������Ȼ��������������________����������������ƹ��壬ֱ����ƽƽ�⣮
��3���ܽ⣺��Ͳ��ȡ�����ˮ������װ���������ƹ�����ձ���ò�����________��ʹ���ܽ⣬����ȴ�����£�
��4���洢�������ƺõ���Һװ���Լ�ƿ��������Ƥ����________���ŵ�ָ���ĵط���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�Ȳ����ڻ��Ϸ�Ӧ�ֲ����ڷֽⷴӦ���ǣ� ��
A. þ+����![]() ����þ B. ��Ȳ+����
����þ B. ��Ȳ+����![]() ������̼+ˮ
������̼+ˮ
C. һ����̼+����![]() ������̼ D. þ+���������þ+����
������̼ D. þ+���������þ+����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��ʵ����������غͶ������̻������ȡ��������ַ�Ӧ���Է�Ӧ��Ĺ������ʽ��л������ã�ʵ�������������ش��������⣺
ʵ��ǰ | ʵ��� |
�Թ��ڹ������ʵ����� | �Թ��ڹ������ʵ����� |
18.0g | 13.2g |
��1���õ��������ٿ�________��
��2������ܻ��ն������̶��ٿ�________��
��3�����պ��һ�����ʿ��������ϣ�������______�ʣ�Ҫ���շ�Ӧ���������Ҫ�õ���������Щ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС���ͬѧ������ͼװ�ý���ʵ�飮
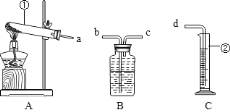
![]() ʵ��Ŀ�ģ����ø��������ȡһƿ��������ϸ��˿ȼ�յ�ʵ�飮
ʵ��Ŀ�ģ����ø��������ȡһƿ��������ϸ��˿ȼ�յ�ʵ�飮
�ڴ��Բⶨ����һ�������ĸ���������ռ��������������
![]() ��Ӧԭ�������ȸ����������������ѧ����ʽΪ________��
��Ӧԭ�������ȸ����������������ѧ����ʽΪ________��
![]() ������ʶ��д����ͼ�������١��ڵ����ƣ���________����________��
������ʶ��д����ͼ�������١��ڵ����ƣ���________����________��
![]() װ�����ӣ�Ϊ�ﵽʵ��Ŀ�Ģڣ���װ�õ���ȷ����˳���ǣ���ӿڵ���ĸ����
װ�����ӣ�Ϊ�ﵽʵ��Ŀ�Ģڣ���װ�õ���ȷ����˳���ǣ���ӿڵ���ĸ����![]() ________
________![]() ________
________![]()
![]() �����ʵ����̷���
�����ʵ����̷���![]() ƿ�е�ˮ����ˣ�ԭ����________��
ƿ�е�ˮ����ˣ�ԭ����________��
�����ռ�����������ϸ��˿ȼ�յ�ʵ��ʱ������ƿ��ը�ѣ�����ʧ��IJ���ԭ�������________��
�۸��ݸ�����ص����������������������Ϊ����ֵ�����ʵ�ʲ�������������������ֵ������Ϊԭ�������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��һ�����п̶Ⱥͻ����ɻ����IJ��������������п����������İ��ף���������ʢ�з�ˮ���ձ��Ϸ�������ʵ�飮�������ͼ��ʾʵ�鱨��
ʵ��Ŀ�� | ʵ������ | ʵ����� |
�ⶨ������____________��������� | �����Ż�ȼ�գ����������ƣ������ƣ����ͣ�ڿ̶�ԼΪ____________��λ���ϣ� | �����ijɷְ�������㣬����Լռ____________�� |
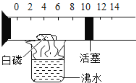
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������á�![]() ���͡�
���͡�![]() ���ֱ��ʾ��ͬ��ԭ�ӣ���ͼ��ʾ��ijЩ��������ʾ��ͼ������������ص�����ĸ�ش�
���ֱ��ʾ��ͬ��ԭ�ӣ���ͼ��ʾ��ijЩ��������ʾ��ͼ������������ص�����ĸ�ش�

(1)���ڴ��������______________��
(2)���ڻ�������________��
(3)��ԭ��ֱ�ӹ��ɵĴ�������________��
(4)�ɷ���ֱ�ӹ��ɵĴ�������________��
(5)ͼ________��ʾ�Ļ�����У���ɸ����ʵ���������࣬һ����________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E�dz��л�ѧ�������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪��A��B��C�����������к�����ߵĽ���Ԫ�أ�A�������C��D��E�����ڼE Ϊ��ɫ��
��1��д����ѧ����ʽC��B��____________________��
��2��D �Ļ�ѧʽ�ǣ�________��
��3��C��һ����;��________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��
��![]() ��
��![]() �գ����϶��������������Ƿ��������ɡ���������������ǿ�����ػ������ɹ�����������ȼ��֮һΪ�����������������ڿ�����ȼ�գ�������������ʵ���ǣ� ��
�գ����϶��������������Ƿ��������ɡ���������������ǿ�����ػ������ɹ�����������ȼ��֮һΪ�����������������ڿ�����ȼ�գ�������������ʵ���ǣ� ��

A. ��������ɫ����
B. �����������ձ����ڻ����Ϸ����ձ��ڲ���ˮ������
C. �Ӵ��ձ��е�����
D. ��ʼ������ȼ�գ�ʱ����˾ͻᷢ����ը
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com