
��5�֣���ʦ����ͼ��ʾװ��Ϊͬѧ��������ʵ�飺Aװ�ü���ƿ��װ�������ԼΪ1��1��
����������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᡣ��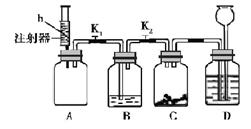
��1���رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A�к���Һ��Ϊ��ɫ��B�г���������Һ�档��ش�
����a�����Ƕ�����̼����b�� ��Һ���ѧʽ����
����b��ˮ��������a������ ���ѧʽ����
��2������K1����״̬������K2��һ��ʱ���ر�K2�����������У��۲쵽D�е������� �����ţ���2�֣���
A�����ƿ��Һ���½� B������©����Һ������ C������©���¶�������
д��Cװ���з�Ӧ�Ļ�ѧ����ʽ�� ��
��1����NaOH�������������Լ ��NH3
��2��A C Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��Fe+H2SO4=FeSO4+H2��
�������������(1)�� �رջ���K1��K2����ע�����е���Һb����ƿ�У�����K1������B����Һ����A��˵��װ��A���ٵ�����Һb��ѹǿ���ͣ�����ζ����ҺB����a���������̼��Ӧ���Ӷ�ʹװ��A�ڵ����������٣�ѹǿ���ͣ�����Һ��Ϊ��ɫ�����Կ��ж���b��NaOH�������������Լ��Һ
����b��ˮ��ͬ��Ҫ�γ�ѹǿ�˵������aӦ��������ˮ����ˮ��Һ�ʼ��ԣ����Կ�����NH3
(2)����װ��A��ѹǿ���ͣ�B�г���������Һ�棬����һ����װ��B�ڵ�ѹǿҲ���ͣ���K2��ͬ������װ��C�ڵ�ѹǿ���ͣ����Կ��Բ쵽D�е������ǹ��ƿ��Һ���½�������©���¶������ݣ�ѡAC������װ��D�е�ϡ��������ѹǿ�Ĺ�ϵ��ѹ��Cװ���У����Է�Ӧ�Ļ�ѧ����ʽ�ǣ�Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��Fe+H2SO4=FeSO4+H2��
���㣺NaOH��NH3�Ļ�ѧ���ʣ����ָʾ���ı�ɫ�����ѹǿ�ı仯����Ļ�ѧ����


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��13�֣�A��B��C�ֱ���Zn��Cu��Ag���ֽ����е�һ�֡�Ϊ�˴����ǵĻ�����з���
������A�������B��C���Σ���������ʵ�������
��ش��������⣺
д��������з�����Ӧ�Ļ�ѧ����ʽ ��
A��B��C���ֽ����Ļ��˳���� > > ��
����b����Ҫ�õ��������������� ��
����A�� ���ѧʽ����ͬ����B���������� ��
��13�˵�пͶ�뵽98��ϡ������ǡ����ȫ��Ӧ�������ɵ������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ������������ԣ�����������dz��������������ƶ��ɵIJݻң�����ң�����е���Ҫ�ɷ���ʲô�أ���ѧ��ȤС�������ҵ���Ҫ�ɷֿ�����K2CO3��С���Ա�ּס���������в���̽����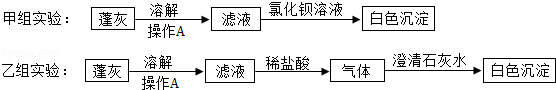
ʵ������еIJ���A�������� ��������Ϊ�� ����ѡ���ס����ҡ�����ʵ�鷽���Ƚ����룮
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ũ����������������������߲�Ʒ�IJ�����������ij���ӵ���������Ӫ��Һ�к���7��08%��KNO3��
��1��KNO3�е�Ԫ�ص����������� ���������һλС������KNO3���� ��ѡ����ʡ������طʡ� �����ʡ����Ϸ��ϡ�)��
��2��ũҵ��������35��4%��KNO3��Һ������150kg��Ӫ��Һ����Ҫˮ������ kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
С��ͬѧ�����г��������ˣ��������Ҫ�ɷ���Fe2O3����������Ҳ�����ˣ������������õ���������ʦ�������������ʦ���ܿ��ó�һƿ�������С����������������һ�£�һ�������������Կ�״��ˣ�С���е��ܾ��棬����ʦ��ʲôԭ����ʦ��ֻ��ʹ�õ�������ͣ�С����������ѧ�Ļ�ѧ֪ʶŪ���ף�����ϸ�������ƿ�ϵ�˵����ԭ������Ҫ�ɷ������ᣬС����ʱ��Ȼ���������û�ѧ����ʽ������ԭ���� ��
С��˼����һ�ᣬǫǫ����ض���ʦ��˵���Ժ�ʹ������������ܹ���ʹ�ã������������������ʦ��Ц�ŵ��ͷ���������û�ѧ����ʽ������ԭ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����̼�����ơ������ǡ�������������������ʣ�ѡ����ʵ������û�ѧʽ��գ�
��1��������Ǧ�����к��е��� ��
��2����Ϊ���幩�ܵ������� ��
��3�����Ƹ��ķ��ͷ۳ɷ�֮һ���� ��
��4������������θ�����ļ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(10��)�Ϻ������㵺���丽���������ҹ������������̲��ŷḻ�ĺ�����Դ��
��1����ˮ���Ρ��Ӻ�ˮ��ȡ�Ĵ����к�����ɳ������þ���Ȼ��Ƶ����ʣ�Ϊ�õ��ϴ����Ȼ��ƣ�����������ˮ��Ȼ��������²�����a���ӹ�����Ba(OH)2��Һ��b�����Թ��������c���ӹ�����Na2CO3��Һ��d�����ˣ�e����������ȷ�IJ���˳���� (����ĸ)����Na2CO3��Һ�������dz�ȥ ��
��2����ˮ��������ͼ��һ��������������ˮ��װ�á�֤���õ���ˮ�ǵ�ˮ�ķ����� ��
��3����ˮ��þ���Ӻ�ˮ����ȡ����þ�Ĺ�������ͼ��ʾ��
������ת�������з����кͷ�Ӧ���� (�Ӧ˳���)����ˮ�б����ͺ����Ȼ�þ����١��������������� ��
��4����ˮ���Ƽ������ƴ�������ͨ����ѧ��Ӧ����NaHCO3��NH4Cl��NaHCO3�ᾧ�������ټ���NaHCO3�Ƶô��
�ٰ���ƴ���������NaHCO3��NH4Cl�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ������NaHCO3��NH4Cl��������Ϊ84��53��5��NaHCO3��NH4Cl���ܽ��������ͼ��ʾ�������NaHCO3�ᾧ������NH4Clû�нᾧ������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ���ѧѧ����ȡ�õijɾ��Լ������������Ĺ��ף����Ϲ���2011�궨Ϊ�����ʻ�ѧ�ꡱ����ش��������⣺
��1�����ϵķ�չ�ƶ����Ľ��������в������ںϳɲ��ϵ��� ������ţ�
| A������ϩ���� | B������� | C���� | D����ë |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣��Դ��Σ���Ҫ�ɷ���NaCl�����в�������ɰ�������Ե�MgCl2��CaCl2�����ʣ�Ϊԭ�ϣ����ʳ��ˮ�����������Ƶļ�Ҫ�������£�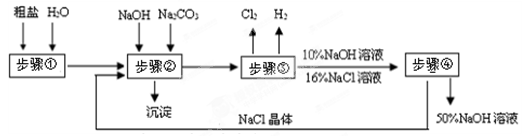
��1������ٳ�ȥ���������ʣ�����Ҫ�������ܽ⡢ ��
��2������ڳ�ȥ���������ʣ�д������һ����ѧ����ʽ ��
��3��д���������һ�ֵ��ʵ����� ����ҵ�Ͽ����ñ������в��������������������ᣬд�������һ����; ��
��4����������Ƶõ�����������ǿ�ҵĸ�ʴ�ԣ��������մ��Ƥ���ϣ�Ҫ ��
��5���������п���ѭ�����õ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com