


 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ζ����Ƭ ��ÿ100g�к����� ������ 2.3g ���� 20g ����ά���� 50mg Ca��P��Fe 120mg ��֬ 1.1g ��ͼ1 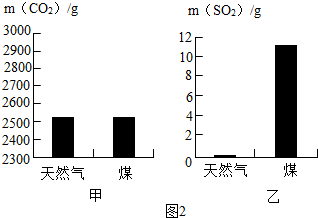 ��1������������ϲ����һ��ʳƷ��ͼ1��ʾ�ǡ�����ζ����Ƭ������ҪӪ���ɷ֣���������Ϊ�����ṩ������Ӫ������ ���� ���� ����ʳ��������ţ�����������彡����������Ӫ���ḻ������ Ӫ���ḻ������ ����2��������ũ�����������Ҫ���ʣ����л��������ڵ��ʵ��� AC AC ��A��NH4Cl B��K2CO3 C��CO��NH2��2 D��Ca3��PO4��2 ��3�������м���ȼ�ϵ����б�Ǩ����������ʶ�˻�ѧ����Ҫ��ֵ�� ú��ľ���Һ��ʯ������ܵ�ú������Ȼ�� ���ȼ��1kg��Ȼ����ú��������CO2��SO2��������ͼ2��ʾ��Ϊ�ˣ�������Ȼ����ȼ�ϵ��ŵ��� ��ֹ�γ����꣬�������� ��ֹ�γ����꣬�������� ����Ȼ������Ҫ�ɷ�CH4��ȼ�յĻ�ѧ����ʽΪCH4+2O2
CH4+2O2 ��
��4���⻯þ��MgH2���������Ϊ�����������Դ�ṩ�����ṩ��ԴʱMgH2��ˮ��Ӧ����һ�ּ����������Ӧ�Ļ�ѧ����ʽΪ MgH2+2H2O=Mg��OH��2+2H2�� MgH2+2H2O=Mg��OH��2+2H2�� ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��2013?��������ѧ�����������ߣ��������ǵ�����ϢϢ��أ� ��1������H��C��O��Na���ֳ�����Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ������ʸ�һ�֣��û�ѧʽ��ʾ���� ���� H2CO3 H2CO3 �����ܲ�������ЧӦ������ CO2 CO2 ���������к����������� H2O H2O ���ܷ��ͷ��е���Ҫ�ɷ�С�մ� NaHCO3 NaHCO3 ����2����ͥ����ʵ������һ����ѧ���磬��ش��������⣺ ���Ȼ�������Ҫ�ĵ�ζƷ�������� ���� ���� ���ɣ�ѡ����ӡ�����ԭ�ӡ������ӡ����ڱ����ζ���еĻ���̿����ȥ�����������ζ�����������˻���̿�� ���� ���� �ԣ��۳���ʱ���е��Ͳ����Ż𣬿��Բ�ȡ������� ���Ϲ��� ���Ϲ��� ���ܿ������ó����е� ʳ�� ʳ�� ��ϴˮ���е�ˮ�����鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��2013?��ɽ����ѧ�����������ߣ��������ǵ�����������ϢϢ��أ������������ʯ���ɱ���С�մ����������У�ѡ����������;��Ӧ�����ʣ���д�ڿհ״��� ��1����������ȼ�ϵ��� ���� ���� ����2�����ͷ۵���Ҫ�ɷ�֮һ��С�մ� С�մ� ����3�������˹�������� �ɱ� �ɱ� ����4�����ڲ�����ͷ�������ò����������ʯ ���ʯ ���鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |