
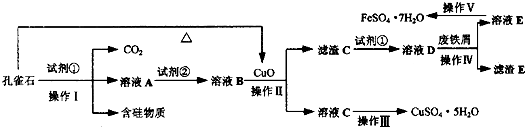
��ȸʯ��Ҫ��Cu2 (OH)2CO3����������Fe��Si�Ļ����ij�����Կ�ȸʯΪ��Ҫԭ���Ʊ�CuSO4��5 H2O�����ײ���G����Ҫ�������£�
��֪�������£�ͨ��������Һ������Զ�ʹFe3+��Fe2+��Cu2+���ɳ�����pH�ֱ����£�
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe(OH)3 | 2.2 | 3.2 |
| Fe(OH)2 | 7.6 | 9.6 |
| Cu(OH)2 | 4.7 | 6.4 |

��1��Cu2(OH)2CO3+2H2SO4��2CuSO4+3H2O+CO2����2�֣�
��2��a��1�֣�
��3��������Һ��PHʱ��Fe3+��Cu2+��ʼ����֮ǰ���ܳ�����ȫ���Ӷ��������ʳ�ȥ��1�֣�
a��1�֣�
��4����Һ�¶ȿ�����80�棬PH������1.5������ʱ��Ϊ4Сʱ���ң�1�֣�
��5��CuO��1�֣� ���ˣ�1�֣�
��6��NH3��1�֣� 2NH3+CO2+CaCl2+H2O==CaCO3��+2NH4Cl��2�֣�
��7��MnO4��+ 5Fe2+ + 8H+="==" Mn2+ + 5Fe3+ + 4H2O��2�֣� 0.0960mol/L��2�֣�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 2.7 | 3.7 |
| Fe2+ | 7.6 | 9.6 |
| Cu2+ | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com