
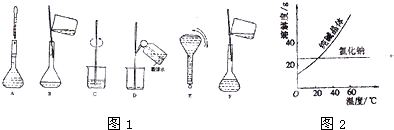
(9��) ʵ��������ȩ��������Һ����������Ӧ��ʵ��ʱ��
��Ϊ������������������Ӧ���� ��Һ��У��������Һ��������ˮ���Թܳ�ϴ�ɾ���
������������Һʱ��ʢ�� ��Һ���Թ�����εμ� ��Һ�ߵα���ֱ�� Ϊֹ���йط�Ӧ�Ļ�ѧ����ʽ�� �� ��
�ۼ���ʱӦ��
���ȣ����������Ļ�ѧ����ʽΪ ��
��ʵ��������������Һʱ����AgNO3��Һ�м��백ˮ�γ�������Һʱ��Ӧ��ֹ���백ˮ�������������������ױ�ը��Ag3N���Լ�����Ҳʧȥ�����ԡ����ƺõ�������Һ���ɾ��ã���������� ������Һ���������ֳ������������ò������β�Ҳ��ֽⷢ�����ҵı�ը��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵�/�� | �е�/�� | �ܶ�/g?cm-3 | �ܽ��� | �Ҷ���C2H6O2 | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� | ������C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| �������� | ���� | �Ҵ� | 1-���� | �������� | ���ᶡ�� |
| �۵㣨�棩 | 16.6 | -117.3 | -89.5 | -83.6 | -73.5 |
| �е㣨�棩 | 117.9 | 78.5 | 117 | 77.06 | 126.3 |
| �ܶȣ�g/cm3�� | 1.05 | 0.79 | 0.81 | 0.90 | 0.88 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A����12�֣�2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ����ͼ���Իش�
��1���صĻ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��2���黯��Ʒ��������������ԭ�ӣ���ɫ����Ϊ ����ͬһ����ԭ����������ԭ�ӹ��ɵĿռ乹��Ϊ ��
��3������˵����ȷ���� ������ĸ����
A���黯��Ʒ���ṹ��NaCl��ͬ
B����һ�����ܣ�As>Ga
C���縺�ԣ�As>Ga
D���黯�ؾ����к�����λ��
E��GaP��GaAs��Ϊ�ȵ�����
��4��N��P��As����ͬһ���壬���⻯��е��ɸߵ��͵�˳���� ��
��5���黯�ؿ��ɣ�CH3��3Ga��AsH3��700��ʱ�Ƶá���CH3��3Ga����ԭ�ӵ��ӻ���ʽΪ ��
B����12�֣�����������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��
CH3COOH+C2H5OHCH3COOC2H5+H2O
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH��
�ڲ����л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34��5 | 78��5 | 117��9 | 77 |
��ش��������⣺
��1��Ũ����������� �����θ���ܵ������� ��
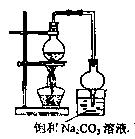
��2������ͼ��ʾװ�����Ʊ�������������������������ƫ�ͣ���ԭ�����Ϊ ��
�ȡ�
��3��ʵ�����õ������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ���ȥ ���ٽ��������ռ�77�����ҵ���֣��Եõ��ϴ���������������
��4��Ŀǰ�Ը÷�Ӧ�Ĵ����������µ�̽����������������������Һ��������˷�Ӧ�Ĵ����������ظ�ʹ�á�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
| ͬһ��Ӧʱ�� | ͬһ��ӦӦ�¶� | ||||
| ��Ӧ�¶�/�� | ת���ʣ�%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���ԣ�%��* |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
| *ѡ����100%��ʾ��Ӧ���ɵIJ���ȫ��������������ˮ |
�ٸ��ݱ������ݣ����� ������ĸ��Ϊ�÷�Ӧ�����������
a.120��,4h b.80��,2h c.60��,4h d.40��,3h
�ڵ���Ӧ�¶ȴﵽ120��ʱ����Ӧѡ���Խ��͵�ԭ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�Ͼ�ʦ���и�����ѧ��ѧ����л�ѧ���� ���ͣ������
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A����12�֣�2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ����ͼ���Իش�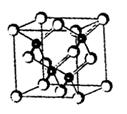
��1���صĻ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��2���黯��Ʒ��������������ԭ�ӣ���ɫ����Ϊ ����ͬһ����ԭ����������ԭ�ӹ��ɵĿ� �乹��Ϊ ��
�乹��Ϊ ��
��3������˵����ȷ���� ������ĸ����
A���黯��Ʒ���ṹ��NaCl��ͬ
B����һ�����ܣ�As>Ga
C���縺�ԣ�As>Ga
D���黯�ؾ����к�����λ��
E��GaP��GaAs��Ϊ�ȵ�����
��4��N��P��As����ͬһ���壬���⻯��е��ɸߵ��͵�˳���� ��
��5���黯�ؿ��ɣ�CH3��3Ga��AsH3��700��ʱ�Ƶá���CH3��3Ga����ԭ�ӵ��ӻ���ʽΪ ��
B����12�֣�����������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��
CH3COOH+C2H5OH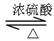 CH3COOC2H5+H2O
CH3COOC2H5+H2O
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH��
�ڲ����л���ķе㣺
| �Լ� | ���� | �Ҵ� | ���� | �������� |
| �е㣨�棩 | 34��5 | 78��5 | 117��9 | 77 |
| ͬһ��Ӧʱ�� | ͬһ��ӦӦ�¶� | ||||
��Ӧ�¶�/ �� �� | ת���ʣ�%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���ԣ�%��* |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
| *ѡ����100%��ʾ��Ӧ���ɵIJ���ȫ��������������ˮ | |||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ������ѧ��ѧ����л�ѧ���� ���ͣ������
��12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A����12�֣�2010���Ϻ������᳡�ݣ��������������ϻ���Ļ��ʹ���˷�������ܣ�LED����Ŀǰ����LEDƷƬ�����ʻ�����GaAs���黯�أ���AlGaInP��������������lnGaN���������أ�Ϊ�����黯�ص�Ʒ���ṹ����ͼ���Իش�
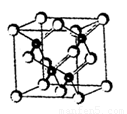
��1���صĻ�̬ԭ�ӵĵ����Ų�ʽ�� ��
��2���黯��Ʒ��������������ԭ�ӣ���ɫ����Ϊ ����ͬһ����ԭ����������ԭ�ӹ��ɵĿռ乹��Ϊ ��
��3������˵����ȷ���� ������ĸ����
A���黯��Ʒ���ṹ��NaCl��ͬ
B����һ�����ܣ�As>Ga
C���縺�ԣ�As>Ga
D���黯�ؾ����к�����λ��
E��GaP��GaAs��Ϊ�ȵ�����
��4��N��P��As����ͬһ���壬���⻯��е��ɸߵ��͵�˳���� ��
��5���黯�ؿ��ɣ�CH3��3Ga��AsH3��700��ʱ�Ƶá���CH3��3Ga����ԭ�ӵ��ӻ���ʽΪ ��
B����12�֣�����������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��
CH3COOH+C2H5OH CH3COOC2H5+H2O
CH3COOC2H5+H2O
��֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH��
�ڲ����л���ķе㣺
|
�Լ� |
���� |
�Ҵ� |
���� |
�������� |
|
�е㣨�棩 |
34��5 |
78��5 |
117��9 |
77 |
��ش��������⣺
��1��Ũ����������� �����θ���ܵ������� ��
��2������ͼ��ʾװ�����Ʊ�������������������������ƫ�ͣ���ԭ�����Ϊ ��
�ȡ�
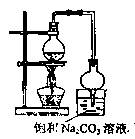
��3��ʵ�����õ������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ���ȥ ���ٽ��������ռ�77�����ҵ���֣��Եõ��ϴ���������������
��4��Ŀǰ�Ը÷�Ӧ�Ĵ����������µ�̽����������������������Һ��������˷�Ӧ�Ĵ����������ظ�ʹ�á�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
|
ͬһ��Ӧʱ�� |
ͬһ��ӦӦ�¶� |
||||
|
��Ӧ�¶�/�� |
ת���ʣ�%�� |
ѡ���ԣ�%��* |
��Ӧʱ��/h |
ת���ʣ�%�� |
ѡ���ԣ�%��* |
|
40 |
77.8 |
100 |
2 |
80.2 |
100 |
|
60 |
92.3 |
100 |
3 |
87.8 |
100 |
|
80 |
92.6 |
100 |
4 |
92.3 |
100 |
|
120 |
94.5 |
98.7 |
6 |
93.0 |
100 |
|
*ѡ����100%��ʾ��Ӧ���ɵIJ���ȫ��������������ˮ |
�ٸ��ݱ������ݣ����� ������ĸ��Ϊ�÷�Ӧ�����������
a.120��,4h b.80��,2h c.60��,4h d.40��,3h
�ڵ���Ӧ�¶ȴﵽ120��ʱ����Ӧѡ���Խ��͵�ԭ�����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com