
ijѧ�����ڱ�������أ� ![]() ��Է�������Ϊ204.0���ⶨδ֪��NaOH��Һ��Ũ�ȣ��ڱ�ʵ���е���ζ��յ�ʱ����Һ��pHԼΪ9.1��
��Է�������Ϊ204.0���ⶨδ֪��NaOH��Һ��Ũ�ȣ��ڱ�ʵ���е���ζ��յ�ʱ����Һ��pHԼΪ9.1��
��1����ѧ����������ƽ�����ڱ����������ʱ�����߾��ŵ�������ֽƬ���������̷�һ��1g���룬ƽ��ʱ����λ����ͼ��ʾ��
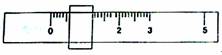
�ɴ˿�֪����ȡ�ڱ���������ص�����Ϊ g.
��2�����ƺõ��ڱ���������ط�����ƿ�У�������ˮ�ܽ⣬��Һ��ɫ���ټ���ָʾ�����Ӽ��ȡ���̪��ʯ����ѡ��1~2�Σ���NaOH��Һ�ζ����յ�ʱ��������
��
��3����ѧ����������ʵ�飬��ȡ������������ͬ����������������Һ��������±���
| ʵ���� | NaOH��Һ�������mL�� |
| 1 | 18.2 |
| 2 | 17.1 |
| 3 | 16.9 |
�ζ������ϴ���ǵ� ��ʵ�飻���NaOH��Һ���ƫ������ԭ�����У�����ţ� ��
��δ������������Һϴ�ӵζ��ܢڵζ�ǰƽ�ӣ��ζ����� �۵ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ �ܵζ�ǰ����ƿ���ڱ������������Һ��ϴ
��4��NaOH��Һ�����ʵ���Ũ��Ϊ��ֻ�г�����ʽ�������������� mol/L��
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com