
 ��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��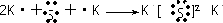
| 1.2L |
| 22.4L/mol |
| ||
| 3 |
| 0.96 | ||||
|
| 1.2L |
| 22.4L/mol |
| ||
| 3 |
| 0.96 | ||||
|
 ��
��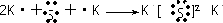 ��
��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��� ��1������A��B��C�������������ģ����ͼ��
��� ��1������A��B��C�������������ģ����ͼ��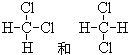 ����
����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡʵ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��12�֣�
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5�� D�ĵ��ʸ����ᷴӦ������D3����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��д��Ԫ�ط��ţ�
A ��C ��E ��
�� B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��
��
��д��E2C�ĵ���ʽ�� ��
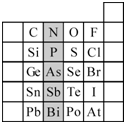
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�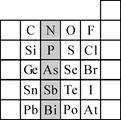
����Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ�� ���ڵ� �塣
����Ԫ�������ɣ�Ԥ�⣺����ǿ�� H3AsO4 H3PO4�����á�>��������ʾ��
��Ԫ��S��������ۺ�����۵Ĵ�����Ϊ____________����һ�������£�S��H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ��������(ѡ���������С������ͬ��)
�� Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ����Һ�д��ڵ���Ҫ������________________________________________________��
������˵����ȷ����
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��1������A��B��C��D��E����ԭ����������������Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��D�ĵ��ʸ����ᷴӦ������D3����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
�� д��Ԫ�ط��ţ�A ��C ��E ��
�� B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��
��
�� д��E2C�ĵ���ʽ�� ��
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�

�� ��Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ�� ���ڵ� �塣
����Ԫ�������ɣ�Ԥ�⣺����ǿ�� H3AsO4 H3PO4�����á�>��������ʾ��
�� Ԫ��S��������ۺ�����۵Ĵ�����Ϊ____________����һ�������£�S��H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ������ ��(ѡ���������С������ͬ��)
�� Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ����Һ�д��ڵ���Ҫ������________________________________________________��
�� ����˵����ȷ����
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��12�֣�
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5�� D�ĵ��ʸ����ᷴӦ������D3����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
�� д��Ԫ�ط��ţ�
A ��C ��E ��
�� B��D������������Ӧˮ�������Ӧ�����ӷ���ʽΪ��
��
�� д��E2C�ĵ���ʽ�� ��
��2��Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�
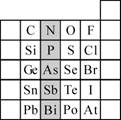
�� ��Ӱ����Ԫ��N��Ԫ�����ڱ��е�λ��Ϊ�� ���ڵ� �塣
����Ԫ�������ɣ�Ԥ�⣺����ǿ�� H3AsO4 H3PO4�����á�>��������ʾ��
�� Ԫ��S��������ۺ�����۵Ĵ�����Ϊ____________����һ�������£�S��H2��Ӧ��һ����(������Ϊ��Ӧ���еij̶�)�����жϣ�����ͬ������Se��H2��Ӧ���ȱ�S��H2��Ӧ�� ������ ��(ѡ���������С������ͬ��)
�� Br2���н�ǿ�������ԣ�SO2���н�ǿ�Ļ�ԭ�ԣ���SO2����ͨ����ˮ����Һ�д��ڵ���Ҫ������________________________________________________��
�� ����˵����ȷ����
A��C��N��O��F��ԭ�Ӱ뾶����ԭ���������������С
B��Si��P��S��ClԪ�صķǽ��������ź˵���������Ӷ���ǿ
C���ɱ�������Һ̬ˮת��Ϊ��̬��Ҫ�˷������ڵĹ��ۼ�
D��HF��HCl��HBr��HI�����ȶ������μ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com