
�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�ء��ס�������ͬһ���壬���������촦��ͬһ���ڣ���ԭ�ӵ������������Ǽס��ҡ���ԭ������������֮�͡��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ�õ�һ�ְ���Y��һ��ǿ��Z�����ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������L,Ҳ����Z��ˮ��Һ��Ӧ�����Σ����������ɻ�����M����ش���������
(1)�����ӵĽṹʾ��ͼΪ_______________��
(2)д��Y�ĵ���ʽ�� ______���õ���ʽ��ʾZ���γɹ��̣�____________________
_____________________________________________________________________��
(3)д����������������ˮ�������������������ˮ���ﷴӦ�����ӷ���ʽ��___________ __________________________________________________________��
(4)��ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2:4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ________��
����֪ʶ�㣺��ԭ�ӽṹ��Ԫ�ؼ��仯����������Ʋ����ʡ����ӵĽṹʾ��ͼ������ʽ����д���Լ��õ���ʽ��ʾ���ۻ�������γɹ��̡�������ԭ��Ӧ�У����õ����غ�ļ��㡣
1��Cl����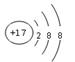
��2��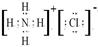
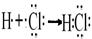
��3��Al��OH��3��OH��=2AlO-��2H2O�� ��4��2��3
��������������������Ϣ�ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ���������Ʋ�XΪNH3, ��ϼס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�ؿ��Ƴ���ΪH����ΪN���ٽ�ϼס�����ͬһ���壬������������ͬһ���ڣ��Ƴ���ΪNa������Ϊ��ԭ�ӵ������������Ǽס��ҡ���ԭ������������֮�ͣ����Ƴ���ԭ�ӵ�������������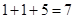 ����֪�����ȣ������ӵĽṹʾ��ͼΪ
����֪�����ȣ������ӵĽṹʾ��ͼΪ �����Ž����ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ�õ�һ�ְ���Y��һ��ǿ��Z����Ӧ����ʽ��3Cl2��4MH3=2NH4Cl��4HCl��N2�����Ƴ�YΪNH4Cl�������ʽ
�����Ž����ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ�õ�һ�ְ���Y��һ��ǿ��Z����Ӧ����ʽ��3Cl2��4MH3=2NH4Cl��4HCl��N2�����Ƴ�YΪNH4Cl�������ʽ ��ZΪHCl���õ���ʽ��ʾZ���γɹ���Ϊ
��ZΪHCl���õ���ʽ��ʾZ���γɹ���Ϊ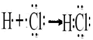 �����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2:4����Ϊ2mol��4mol,��Ϸ�Ӧ����ʽ��֪��2molNH4Cl�����������������2mol MH3��������ʧȥ��6mol���ӣ�����3molCl2����ԭ����˷�Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ2��3��
�����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2:4����Ϊ2mol��4mol,��Ϸ�Ӧ����ʽ��֪��2molNH4Cl�����������������2mol MH3��������ʧȥ��6mol���ӣ�����3molCl2����ԭ����˷�Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ2��3��
����϶��ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������L,Ҳ����Z��ˮ��Һ��Ӧ�����Σ���֪LΪNaOH���Ƴ���ΪAl�����������ӷ���ʽ��Al��OH��3��OH��=2AlO-��2H2O����
���㣺Ԫ�����ڱ�Ԫ�ص��ƶϼ������ʵĿ���
������Ԫ�ص��ƶ�������߿����ȵ����ص㣬����һ�����Ѷȣ����ؿ���ѧ����Ԫ����Ԫ�����ڱ���λ����Ǻ����ʵ����ճ̶ȣ�ͬʱ����ѧ������˼ά��������ɢ˼ά�������������⣬�����������������Ĺؼ�����Ҫ�����ɵľ�����Ϣ������ƽ�����������յ�Ԫ�����ʣ������ƶϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
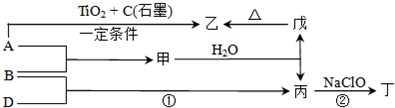
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| ���¸�ѹ |
| ���� |
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
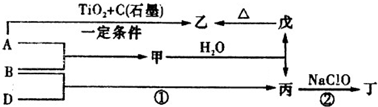
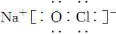
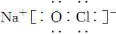
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


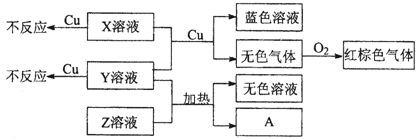
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Ӱ뾶�����������ң��� | B����̬�⻯����ȶ��ԣ��ף��� | C��������γɵĻ��������һ�� | D�����������������������Ӧ��ˮ����֮���������ܷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com