
ЁОЬтФПЁПвбжЊФГШмвКXПЩФмгЩK+ЁЂMg2+ЁЂCu2+ЁЂAg+ЁЂBa2+ЁЂAl3+ЁЂFe2+ЁЂ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() ЁЂI-ЁЂ
ЁЂI-ЁЂ![]() ЁЂ
ЁЂ![]() жаЕФШєИЩжжРызгзщГЩЁЃФГЛЏбЇаЫШЄаЁзщЭЈЙ§ЯТСаЪЕбщШЗЖЈСЫЦфзщГЩЁЃ
жаЕФШєИЩжжРызгзщГЩЁЃФГЛЏбЇаЫШЄаЁзщЭЈЙ§ЯТСаЪЕбщШЗЖЈСЫЦфзщГЩЁЃ
(1)ИљОнЯТСаЪЕбщВНжшКЭЯжЯѓЃЌЭЦЖЯЪЕбщНсТлЃК
ЪЕбщВНжшгыЪЕбщЯжЯѓ | ЪЕбщНсТл |
Ђё.ЙлВьШмвКЃКЮоЩЋЭИУї | ЂйдШмвКжавЛЖЈВЛКЌЕФРызгЪЧ____ЁЃ |
Ђђ.ШЁЪЪСПИУШмвКЃЌМгШыЙ§СПЕФЯѕЫсЃЌгаЦјЬхЩњГЩЃЌВЂЕУЕНЮоЩЋШмвК | ЂкдШмвКжавЛЖЈВЛКЌЕФРызгЪЧ_____ЃЌвЛЖЈКЌгаЕФРызгЪЧ____ЁЃ |
Ђѓ.дкЂђЫљЕУШмвКжадйМгШыЙ§СПЕФЬМЫсЧтяЇШмвКЃЌгаЦјЬхЩњГЩЃЌЭЌЪБЮіГіАзЩЋГСЕэA | ЂлдШмвКжаЛЙвЛЖЈКЌгаЕФРызгЪЧ____ЃЌЩњГЩГСЕэAЕФРызгЗНГЬЪНЮЊ____ЁЃ |
Ђє.дкЂѓЫљЕУШмвКжадйж№ЕЮМгШыЧтбѕЛЏБЕШмвКжСЙ§СПЃЌМгШШвВгаЦјЬхЩњГЩЃЌЭЌЪБЮіГіАзЩЋГСЕэB | ЂмАзЩЋГСЕэBжавЛЖЈКЌга___ЃЌПЩФмКЌга____ЁЃ |
(2)ЩЯЪіЪЕбщВНжшЂєжаПЊЪМНзЖЮвЛЖЈЗЂЩњЕФРызгЗНГЬЪНЪЧ______ЁЃ
(3)ИУЛЏбЇаЫШЄаЁзщЕФЭЌбЇЮЊСЫНјвЛВНШЗЖЈBЕФГЩЗжЃЌШЁвЛЖЈСПОЯДЕгКѓЕФBгыYШмвКЗДгІЃЌАзЩЋЙЬЬхЕФЮяжЪЕФСПгыYШмвКЬхЛ§жЎМфЕФЙиЯЕШчЭМЫљЪОЁЃ

YПЩФмЮЊ(ЬюЛЏбЇЪН)____ЃЌBЕФзщГЩЮЊ______ЁЃ
ЁОД№АИЁПCu2+ЁЂFe2+ЁЂ![]() I-ЁЂ
I-ЁЂ![]() ЁЂMg2+ЁЂAg+ЁЂBa2+ЁЂAl3+
ЁЂMg2+ЁЂAg+ЁЂBa2+ЁЂAl3+ ![]() ЁЂK+
ЁЂK+ ![]() Al3+ЃЋ3
Al3+ЃЋ3![]() ===Al(OH)3Ё§ЃЋ3CO2Ёќ BaCO3 BaSO4 Ba2+ЃЋ2OH-ЃЋ2
===Al(OH)3Ё§ЃЋ3CO2Ёќ BaCO3 BaSO4 Ba2+ЃЋ2OH-ЃЋ2![]() ===BaCO3Ё§ЃЋ
===BaCO3Ё§ЃЋ![]() ЃЋ2H2O HCl(ЛђHNO3) BaSO4КЭBaCO3ЃЌЧв
ЃЋ2H2O HCl(ЛђHNO3) BaSO4КЭBaCO3ЃЌЧв![]() ЃН
ЃН![]()
ЁОНтЮіЁП
IЃЎЙлВьШмвКЃКЮоЩЋЭИУїЃЌвЛЖЈВЛДцдкгаЩЋРызгЃКCu2ЃЋЁЂ![]() ЁЂFe2ЃЋЃЛ
ЁЂFe2ЃЋЃЛ
ЂђЃЎШЁЪЪСПИУШмвКЃЌМгШыЙ§СПЕФЯѕЫсЃЌгаЦјЬхЩњГЩЃЌИУЦјЬхЮЊЖўбѕЛЏЬМЃЌШмвКжавЛЖЈДцдк![]() ЃЌвЛЖЈВЛДцдкгыCO32ЃРызгЗДгІЕФMg2ЃЋЁЂAgЃЋЁЂBa2ЃЋЁЂAl3ЃЋРызгЃЛЕУЕНЮоЩЋШмвКЃЌвЛЖЈВЛДцдкIЃЁЂ
ЃЌвЛЖЈВЛДцдкгыCO32ЃРызгЗДгІЕФMg2ЃЋЁЂAgЃЋЁЂBa2ЃЋЁЂAl3ЃЋРызгЃЛЕУЕНЮоЩЋШмвКЃЌвЛЖЈВЛДцдкIЃЁЂ![]() ЃЌдйИљОнШмвКГЪЕчжаадХаЖЯдШмвКжавЛЖЈДцдкЮЈвЛбєРызгKЃЋЃЛ
ЃЌдйИљОнШмвКГЪЕчжаадХаЖЯдШмвКжавЛЖЈДцдкЮЈвЛбєРызгKЃЋЃЛ
ЂѓЃЎдкЂђЫљЕУШмвКжадйМгШыЙ§СПNH4HCO3ШмвКЃЌгаЦјЬхЩњГЩЃЌЭЌЪБЮіГіАзЩЋГСЕэAЃЌЫЕУїШмвКжавЛЖЈДцдкТСРызгЃЌдШмвКжавЛЖЈДцдк![]() ЃЛ
ЃЛ
IVЃЎдкЂѓЫљЕУШмвКжаКЌгаЙ§СПЕФЬМЫсЧтяЇЃЌМгШыЙ§СПBa(OH)2ШмвКжСЙ§СПЃЌМгШШЛсгаАБЦјЩњГЩЃЌАзЩЋГСЕэПЩФмЮЊЬМЫсБЕЛђЬМЫсБЕКЭСђЫсБЕЕФЛьКЯЮяЁЃ
(1)IЃЎЙлВьШмвКЃКЮоЩЋЭИУїЃЌвЛЖЈВЛДцдкгаЩЋРызгЃКCu2ЃЋЁЂMnO4ЃЁЂFe2ЃЋЃЛЙЪД№АИЮЊЃКCu2ЃЋЁЂMnO4ЃЁЂFe2ЃЋЃЛ
ЂђЃЎШЁЪЪСПИУШмвКЃЌМгШыЙ§СПЕФЯѕЫсЃЌгаЦјЬхЩњГЩЃЌИУЦјЬхЮЊЖўбѕЛЏЬМЃЌШмвКжавЛЖЈДцдк![]() ЃЌвЛЖЈВЛДцдкгы
ЃЌвЛЖЈВЛДцдкгы![]() РызгЗДгІЕФMg2ЃЋЁЂAgЃЋЁЂBa2ЃЋЁЂAl3ЃЋРызгЃЛЕУЕНЮоЩЋШмвКЃЌвЛЖЈВЛДцдкIЃЁЂ
РызгЗДгІЕФMg2ЃЋЁЂAgЃЋЁЂBa2ЃЋЁЂAl3ЃЋРызгЃЛЕУЕНЮоЩЋШмвКЃЌвЛЖЈВЛДцдкIЃЁЂ![]() ЃЌдйИљОнШмвКГЪЕчжаадХаЖЯдШмвКжавЛЖЈДцдкЮЈвЛбєРызгKЃЋЃЛЙЪД№АИЮЊЃКI-ЁЂ
ЃЌдйИљОнШмвКГЪЕчжаадХаЖЯдШмвКжавЛЖЈДцдкЮЈвЛбєРызгKЃЋЃЛЙЪД№АИЮЊЃКI-ЁЂ![]() ЁЂMg2+ЁЂAg+ЁЂBa2+ЁЂAl3+ЃЛ
ЁЂMg2+ЁЂAg+ЁЂBa2+ЁЂAl3+ЃЛ
ЂѓЃЎдкЂђЫљЕУШмвКжадйМгШыЙ§СПNH4HCO3ШмвКЃЌгаЦјЬхЩњГЩЃЌЭЌЪБЮіГіАзЩЋГСЕэAЃЌЫЕУїШмвКжавЛЖЈДцдкТСРызгЃЌдШмвКжавЛЖЈДцдк![]() ЃЌЗДгІЕФРызгЗНГЬЪНЮЊAl3ЃЋ+3
ЃЌЗДгІЕФРызгЗНГЬЪНЮЊAl3ЃЋ+3![]() =Al(OH)3Ё§+3CO2ЁќЃЌЙЪД№АИЮЊЃК
=Al(OH)3Ё§+3CO2ЁќЃЌЙЪД№АИЮЊЃК![]() ЃЛAl3+ЃЋ3
ЃЛAl3+ЃЋ3![]() =Al(OH)3Ё§ЃЋ3CO2ЁќЃЛ
=Al(OH)3Ё§ЃЋ3CO2ЁќЃЛ
IVЃЎдкЂѓЫљЕУШмвКжаКЌгаЙ§СПЕФЬМЫсЧтяЇЃЌМгШыЙ§СПBa(OH)2ШмвКжСЙ§СПЃЌМгШШЛсгаАБЦјЩњГЩЃЌАзЩЋГСЕэПЩФмЮЊЬМЫсБЕЛђЬМЫсБЕКЭСђЫсБЕЕФЛьКЯЮяЃЌЙЪД№АИЮЊЃКBaCO3ЃЛBaSO4ЃЛ
(2)ЪЕбщЂєжаПЊЪМНзЖЮЗЂЩњЗДгІЮЊЙ§СПЕФЬМЫсЧтяЇгыЧтбѕЛЏБЕЗДгІЃЌЗДгІЕФРызгЗНГЬЪНЮЊЃК2HCO3Ѓ+Ba2ЃЋ+2OH=BaCO3Ё§+2H2O+![]() ЃЌЙЪД№АИЮЊЃК2HCO3Ѓ+Ba2ЃЋ+2OH=BaCO3Ё§+2H2O+
ЃЌЙЪД№АИЮЊЃК2HCO3Ѓ+Ba2ЃЋ+2OH=BaCO3Ё§+2H2O+![]() ЃЛ
ЃЛ
(3)ГСЕэBВПЗжШмвКYЫсЃЌYПЩФмЮЊЯѕЫсЛђбЮЫсЃЌдђBжавЛЖЈКЌгаBaCO3ЁЂBaSO4ЃЌгЩЭМЯѓПЩжЊ![]() =
=![]() ЃЌЙЪД№АИЮЊЃКHClЛђHNO3ЃЛBaCO3ЁЂBaSO4Чв
ЃЌЙЪД№АИЮЊЃКHClЛђHNO3ЃЛBaCO3ЁЂBaSO4Чв![]() =
=![]() ЁЃ
ЁЃ


 ШЋГЬН№ОэЯЕСаД№АИ
ШЋГЬН№ОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСђЫсаПЪЧжЦдьаПБЕАзКЭаПбЮЕФжївЊдСЯЃЌвВПЩгУзїФОВФЕФЗРИЏМСЕШЁЃгУбѕЛЏаПбЬГО(жївЊГЩЗжЮЊZnOЃЌЛЙКЌгаЩйСПPbOЁЂCuOЁЂFe2 O3ЁЂFeOЕШ)ЩњВњZnSO4ЁЄ7H2OЕФСїГЬШчЯТЃК

гаЙиН№ЪєРызг[c(Mn+)= 0.l mol/L]аЮГЩЧтбѕЛЏЮяГСЕэЕФpHЗЖЮЇШчЯТБэЃК
Н№ЪєРы | Fe3+ | Fe2+ | Zn2+ | Cu2+ |
ПЊЪМГСЕэЕФpH | 1.5 | 6.3 | 6.2 | 4.7 |
ГСЕэЭъШЋЕФpH | 2.8 | 8.3 | 8.2 | 6.7 |
(1)ЁАЫсНўЁБЪБгУЕФЯЁЫсЪЧ____ЃЛТЫдќ1жївЊГЩЗжЪЧ____ЁЃ
(2)ЁАбѕЛЏЁБЪБЕФРызгЗНГЬЪНЮЊ_________ЃЛМгШыZnOГ§дгЪБШмвКЕФpHПижЦЗЖЮЇЪЧ____ЁЋ5.0ЁЃ
(3)ТЫдќ3КЌгааПКЭ____ЃЛТЫвКЕУЕНZnSO4ЁЄ7H2OЕФВйзїЪЧ____ЁЂЯДЕгЁЂИЩдяЁЃ
(4)ШЁ14.35gZnSO4ЁЄ7H2OМгШШжСВЛЭЌЮТЖШЃЌЪЃгрЙЬЬхЕФжЪСПШчЯТБэ
ЮТЖШЃЏЁц | 100 | 250 | 680 | 930 |
жЪСП/g | 8. 95 | 8. 05 | 6. 72 | 4.05 |
дђ680ЁцЪБЪЃгрЙЬЬхЕФЛЏбЇЪНЮЊ________(ЬюађКХ)ЁЃ
AЃЎZnO BЃЎZn3O(SO4
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈжаЃЌе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
A.![]() гы
гы![]() ЛЅЮЊЭЌЯЕЮя
ЛЅЮЊЭЌЯЕЮя
B.ЗжзгзщГЩЮЊC4H8O2ЃЌЦфжаЪєгкѕЅЕФНсЙЙга4жж
C.ввДМКЭввУбЛЅЮЊЭЌЗжвьЙЙЬх
D.ОпгаЯрЭЌЕФЗжзгЭЈЪНЕФгаЛњЮявЛЖЈЪЧЭЌЯЕЮя
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдМзБНЮЊдСЯКЯГЩФГжжЪГгУЯуСЯ(гаЛњЮяG)КЭФГжжжЮСЦЗЮНсКЫвЉЮяЕФгааЇГЩЗж(гаЛњЮяPASNa)ЕФТЗЯпШчЯТЃК
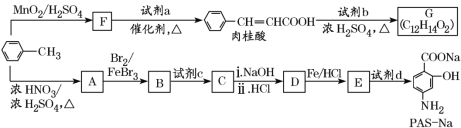
вбжЊЃКЂй
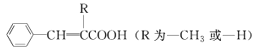
Ђк![]()
Ђл![]()
ЛиД№ЯТСаЮЪЬтЃК
(1)ШтЙ№ЫсжаЫљКЌЙйФмЭХЕФУћГЦЪЧ______________ЁЃ
(2)ЂйЪдМСaЕФНсЙЙМђЪНЪЧ________________ЁЃ
ЂкаДГігЩAЩњГЩBЕФЛЏбЇЗНГЬЪНЃК____________________ЁЃ
(3)вбжЊЪдМСbЮЊЯрЖдЗжзгжЪСПЮЊ60ЕФДМЃЌЧвЮожЇСДЃЌаДГіGЕФНсЙЙМђЪНЃК________________ЃЌгЩШтЙ№ЫсжЦШЁGЕФЗДгІРраЭЪЧ________ЁЃ
(4)ЂйЕБЪдМСdЙ§СПЪБЃЌПЩвдбЁгУЕФЪдМСdЪЧ_____(ЬюзжФИ)ЁЃ
aЃЎNaOHЁЁЁЁbЃЎNa2CO3ЁЁЁЁcЃЎNaHCO3
ЂкВЮееЬтжааХЯЂЃЌЩшМЦвд![]() ЮЊЦ№ЪМдСЯжЦБИ
ЮЊЦ№ЪМдСЯжЦБИ ЕФКЯГЩТЗЯпЁЃ
ЕФКЯГЩТЗЯпЁЃ
____________________ЁЃ
(5)дкШтЙ№ЫсЗжзгжаЬМЬМЫЋМќДпЛЏМгЧтКѓЕУЕНЛЏКЯЮяX(ЗжзгЪНЮЊC9H10O2)ЃЌXгаЖржжЭЌЗжвьЙЙЬхЃЌЗћКЯЯТСаЬѕМўЕФга________жжЁЃаДГіЦфжавЛжжДІгкЖдЮЛЧвКЫДХЙВеёЧтЦзжаБШР§ЮЊ6ЃК1ЃК2ЃК2ЃК1ЕФНсЙЙМђЪН______________________________ЁЃ
aЃЎБНЛЗЩЯгаСНИіШЁДњЛљ
bЃЎФмЗЂЩњвјОЕЗДгІ
cЃЎгыNaзїгУгаH2ВњЩњ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊIЃЁЂFe2ЃЋЁЂSO2ЁЂClЃКЭH2O2ОљгаЛЙдадЃЌЫќУЧдкЫсадШмвКжаЛЙдадЕФЫГађЮЊSO2>IЃ>Fe2ЃЋ>H2O2>ClЃЃЌдђЯТСаЗДгІВЛПЩФмЗЂЩњЕФЪЧ
A.![]()
B.![]()
C.![]()
D.![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЭЌбЇдкЪЕбщЪвНјаа1ЃЌ2-ЖўфхввЭщЕФЯћШЅЗДгІЃЌАДЯТСаВНжшНјааЃЌЧыЬюПе:

(1)АДЭМСЌНгКУвЧЦїзАжУВЂ___ЁЃ
(2)дкЪдЙмaжаМгШы2 mL 1ЃЌ2-ЖўфхввЭщКЭ5 mL 10% NaOHЕФ___ШмвКЃЌдйЯђЪдЙмжаМгШыМИЦЌ___ЁЃ
(3)дкЪдЙмbжаМгЩйСПфхЫЎЁЃ
(4)гУЫЎдЁЗЈМгШШЪдЙмРяЕФЛьКЯЮяЃЌГжајМгШШвЛЖЮЪБМфКѓЃЌАбЩњГЩЕФЦјЬхЭЈШыфхЫЎжаЃЌЙлВьЕНЕФЯжЯѓЪЧ____ЁЃ
(5)ЂйаДГіЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК__________ЁЃ
ЂкБОЪЕбщжагІзЂвтЕФЮЪЬтга______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЦћГЕЮВЦјОЛЛЏдРэЮЊ2NO(g)+2CO(g)N2(g)+2CO2(g)ІЄHЃНЃ746.5kJЁЄmolЃ1ЃЌШчЭМЮЊдкВЛЭЌГѕЪМХЈЖШЕФCOКЭВЛЭЌДпЛЏМСЂёЁЂЂђзїгУЯТ(ЦфЫћЬѕМўЯрЭЌ)ЃЌЬхЛ§ЮЊ2LЕФУмБеШнЦїжаn(N2)ЫцЗДгІЪБМфЕФБфЛЏЧњЯпЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ( )

A.aЕуЪБЃЌДпЛЏМСЂёЁЂЂђзїгУЯТCOЕФзЊЛЏТЪЯрЕШ
B.0ЁЋ6hФкЃЌДпЛЏМСЂёЕФДпЛЏаЇЙћБШДпЛЏМСЂђЕФКУ
C.0ЁЋ5hФкЃЌДпЛЏМСЂёзїгУЯТCOЕФЗДгІЫйТЪЮЊ0.32molЁЄLЃ1ЁЄhЃ1
D.0ЁЋ12hФкЃЌДпЛЏМСЂђзїгУЯТЗДгІЗХГіЕФШШСПБШДпЛЏМСЂёЕФЖр
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаЛњЮя![]() ЭЌЪБТњзуЯТСаЬѕМўЃКЂйКЌБНЛЗЧвВЛКЌМзЛљЃЛЂкБНЛЗЩЯвЛТШШЁДњЮяжЛ2жжЃЛЂл
ЭЌЪБТњзуЯТСаЬѕМўЃКЂйКЌБНЛЗЧвВЛКЌМзЛљЃЛЂкБНЛЗЩЯвЛТШШЁДњЮяжЛ2жжЃЛЂл![]() гызуСПЕФ
гызуСПЕФ![]() ЗДгІЩњГЩ
ЗДгІЩњГЩ![]() ЃЛЂмгі
ЃЛЂмгі![]() ШмвКВЛЯдЩЋ.AШчЭМЫљЪОзЊЛЏЙиЯЕЃК
ШмвКВЛЯдЩЋ.AШчЭМЫљЪОзЊЛЏЙиЯЕЃК
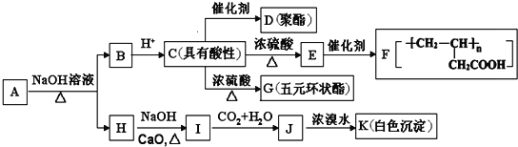
вбжЊЃК![]()
ЛиД№ЯТСаЮЪЬтЃК
(1)EжаЙйФмЭХУћГЦ_____ЃЌHЕФЗжзгЪНЮЊ_______.
(2)гЩCЩњГЩGЕФЗДгІРраЭЪЧ________.
(3)AЕФНсЙЙМђЪНЮЊ____ЃЌGЕФНсЙЙМђЪНЮЊ_________.
(4)ЂйаДГіCDЗДгІЕФЛЏбЇЗНГЬЪН_________ЃЛ
ЂкаДГіIЁњJЗДгІЕФРызгЗНГЬЪН_____________.
(5)CЕФЭЌЗжвьЙЙЬхжаФмЭЌЪБТњзуЯТСаЬѕМўЃКa.ФмЗЂЩњвјОЕЗДгІЃЌb.ФмЗЂЩњдэЛЏЗДгІЃЛc.Фмгы![]() ЗДгІВњЩњ
ЗДгІВњЩњ![]() ЃЌЙВга_____жж(ВЛКЌСЂЬхвьЙЙ).ЦфжаКЫДХЙВеёыБЦзЯдЪОЮЊ3зщЗхЃЌЧвЗхУцЛ§БШЮЊ6:1:1ЕФЪЧ_________(аДНсЙЙМђЪН).
ЃЌЙВга_____жж(ВЛКЌСЂЬхвьЙЙ).ЦфжаКЫДХЙВеёыБЦзЯдЪОЮЊ3зщЗхЃЌЧвЗхУцЛ§БШЮЊ6:1:1ЕФЪЧ_________(аДНсЙЙМђЪН).
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. 1 molБНМзЫсдкХЈH2SO4ДцдкЯТгызуСПввДМЗДгІПЩЕУ1 molБНМзЫсввѕЅ
B. ЖдБНЖўМзЫс(![]() )гыввЖўДМ(HOCH2CH2OH)ФмЭЈЙ§МгОлЗДгІжЦШЁОлѕЅЯЫЮЌ(
)гыввЖўДМ(HOCH2CH2OH)ФмЭЈЙ§МгОлЗДгІжЦШЁОлѕЅЯЫЮЌ(![]() )
)
C. ЗжзгЪНЮЊC5H12OЕФДМЃЌФмдкЭДпЛЏЯТБЛO2бѕЛЏЮЊШЉЕФЭЌЗжвьЙЙЬхга4жж
D. ![]() ЗжзгжаЕФЫљгадзггаПЩФмЙВЦНУц
ЗжзгжаЕФЫљгадзггаПЩФмЙВЦНУц
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com