
 �屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�У�
�屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�У�| Fe |
| Fe |
| Fe |
| Fe |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���֣��屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽 �������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��C��ʢ��CCl4�������� ��
��3����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м��� ��Һ�������� ������֤�����������Թ�D�м��� ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
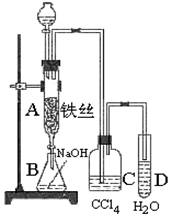
�屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽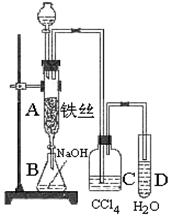 �������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��C��ʢ��CCl4�������� ��
��3����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м��� ��Һ�������� ������֤�����������Թ�D�м��� ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣��屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��C��ʢ��CCl4�������� ��
��3����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м��� ��Һ�������� ������֤�����������Թ�D�м��� ��Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣��屽��һ�������ϳ�ҽҩ��ũҩ����Ҫԭ�ϣ�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬��A�¶˻����رյ�ǰ���£��ٽ����Һ�������뷴Ӧ��A�С�
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��2��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��C��ʢ��CCl4�������� ��
��3����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м��� ��Һ�������� ������֤�����������Թ�D�м��� ��Һ��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com