
����Ŀ��ClO2��NaClO2������Ư���ԣ���ҵ����ClO2������NaClO2�Ĺ���������ͼ��ʾ��
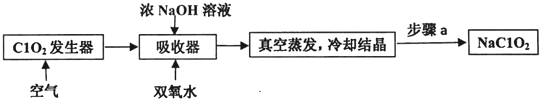
����˵������ȷ����( )
A.����a�IJ����������ˡ�ϴ�Ӻ���
B.ͨ�������Ŀ�������ϳ�ClO2��ʹ�䱻�������������
C.��ҵ�Ͽɽ�ClO2�Ƴ�NaClO2���壬�������������
D.������������NaClO2�����ӷ���ʽ��2ClO2+H2O2=2C1O2- -+O2��+2H+
���𰸡�D
��������
�����̿�֪��������ͨ��������ɽ�ClO2�ų���ȷ���䱻������գ����������з���2ClO2+2NaOH+H2O2�T2NaClO2+O2+2H2O��Ȼ�������������ȴ�ᾧ���پ������ˡ�ϴ�Ӻ���õ�NaClO2���Դ˽����⡣
A������a�ǽ��������Һ��ȡ����Ȼ�������Բ����������ˡ�ϴ�Ӻ��A��ȷ��
B����Ӧ�����������к�������ClO2��ͨ�������Ŀ������ClO2�ݳ���������������ŨNaOH��Һ��˫��ˮ������գ�B��ȷ��
C����ҵ�����û�ԭ����NaOH��Һ����ClO2�Ƴ�NaClO2���壬�����Ϊ�ȶ���������������䣬C��ȷ��
D����������Ӧ�ڼ��������½��У�����NaClO2�����ӷ���ʽ��2ClO2+H2O2+2OH-=2C1O2-+O2��+2H2O��D����ȷ��
��ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ��ѡ��3�����ʽṹ������]
��̼���ء��顢���Ļ����ﱻ�㷺Ӧ�����²����Ʊ�������������������������ʽṹ���֪ʶ���ش��������⣺
��1�����������ء��顢������Ԫ���У���һ�������ɴ�С˳��Ϊ___________���縺���ɴ�С˳��Ϊ___________����Ԫ�ط�����д��
��2��Ԫ�ص�����ͬ�塣��̬��Ԫ�صļ۵����Ų�ʽΪ______��Ԥ������⻯����ӵ�����ṹΪ_____����е��NH3��_____�������������������������ж�������_________��
��3��������Ӧ�ù㷺��
��������ԭ�ӵ����Ų�ͼ��ʾ��״̬�У�������ͺ���ߵķֱ�Ϊ___________��___________�����ţ�
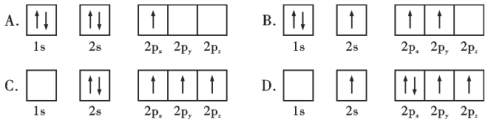
�ھ����������Է����ֳ�����ʮ����Ķ��������Σ��������ʳ�Ϊ�����___________��
����֪BP���۵�ܸߣ��侧���ṹ����ͼ��ʾ��
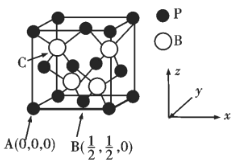
C���ԭ������Ϊ___________��Bԭ����Pԭ�ӵ��������Ϊdpm��������ܶ�Ϊpg/cm3��NA���������ӵ�������ֵ������=___________g/cm3���ú�d��NA�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��г���5��Ԫ�������ڱ��е�λ�á�
�� ���� | ��A | 0 | ||||||
1 | ��A | ��A | ��A | ��A | ��A | ��A | ||
2 | �� | �� | ||||||
3 | �� | �� | �� |
(1)�ٵ�Ԫ�ط�����_______���ݵ�ԭ�ӽṹʾ��ͼ��__________��
(2)����Ԫ���У��ǽ�������ǿ����___________(��Ԫ�ط���)��
(3)��Ԫ�ص�����������Ӧ��ˮ�����______��(������������������������)��
(4)�ۢܢ�����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳����______(��Ԫ�ط���)��
(5)Ԫ�آ���Ԫ�آڵĵ��������Խ�ǿ����________(�ѧʽ)����
(6)Ԫ�آܵ������������Ԫ�آ۵�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij̼������Һ����μ�1 mol��L��1�����ᣬ�����Һ��Cl����HCO3-�����ʵ����������������Ĺ�ϵ��ͼ��ʾ������n2��n1��3��2��������˵������ȷ����
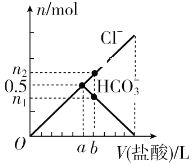
A. b�����ֵΪ0.6
B. ��̼������Һ�к���1 mol Na2CO3
C. b��ʱ����CO2�����ʵ���Ϊ0.3 mol
D. oa�η�Ӧ�����ӷ���ʽ��ab�η�Ӧ�����ӷ���ʽ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ƺͼ��Ǽ����õĽ���Ԫ�أ��ƺͼؼ����������������������й㷺��Ӧ�á�
��1��д�����ֿ���ʳ�õĺ��ƻ�����Ļ�ѧʽ��________����0.01 mol��������(��Na2O2����Na2O����Na2CO3����NaCl)�ֱ����100 mL����ˮ�У��ָ������£�������Һ��������Ũ���ɴ�С��˳����(��Һ����仯���Բ���)_______��
��2�����ڼر��Ƹ����ã��Ʊ�K2Oһ�����õ��ʼػ�ԭ��Ӧ�Ĺ�����������λ��������Σ���д���ü�������ط�Ӧ��ȡK2O�Ļ�ѧ����ʽ(����һ�ֵ�������)��______________________��K2O2Ҳ��ǿ�����ԣ���д������SO2������Ӧ�Ļ�ѧ����ʽ��_______________________��
��3��ijѧ����Na2CO3�� KHCO3��ɵ�ij��������ʵ�飬����������(��������ʵ���Ũ������Ҳ�����HCl�Ļӷ�)
ʵ����� | �� | �� | �� | �� |
�������/mL | 50 | 50 | 50 | 50 |
��������/g | 3.06 | 6.12 | 9.18 | 12.24 |
�����������/L(���) | 0.672 | 1.344 | 1.568 | 1.344 |
�������ݼ���������������ʵ���Ũ��Ϊ__________��ԭ�������Ʒ��n(Na2CO3)��n(KHCO3)��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����̷��з��ֵĻ���ԭ�������谷���������з�Ӧ�ϳɣ�CaO��3C ![]() CaC2��CO����CaC2��N2
CaC2��CO����CaC2��N2 ![]() CaCN2��C��CaCN2��2H2O=NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
CaCN2��C��CaCN2��2H2O=NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
��1��д����Ca��ͬһ������δ�ɶԵ��������Ļ�̬ԭ�ӵĵ����Ų�ʽ��___________��CaCN2��������ΪCN22-����CN22-��Ϊ�ȵ�����ķ�����N2O���ɴ˿�����֪CN22-�Ŀռ乹��Ϊ________��
��2��1mol���ط���[CO��NH2��2]�к��е���������������Ŀ֮��Ϊ_______��
��3�������谷�׳�������������ṹΪ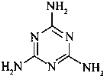 �����е�ԭ�ӵ��ӻ���ʽ��_____________��
�����е�ԭ�ӵ��ӻ���ʽ��_____________��
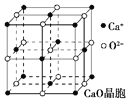
��4��CaO������ͼ��ʾ��CaO������Ca2������λ��Ϊ______��Ca2����ȡ�Ķѻ���ʽΪ____________������O2-����Ca2���ѻ��γɵİ������϶�У���֪CaO������ܶ�Ϊ�������о������������������֮��ľ���_____________���г�����ʽ����
��5����λ������K3[Fe(CN)n]���������ӻ������ɫ��������˿����ڼ����������ӣ���֪��ԭ�ӵ������������������ṩ������֮��Ϊ14����n=_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ҡ������ˮ�����DZ����ˮ��Դ�������̲��ŷḻ�Ļ�ѧ��Դ��
I.��1����ˮ�����ô�����ͨ����Ca2+��Mg2+��SO42-�����ʣ�Ϊ�˵õ����Σ������Լ�������Ⱥ�˳����ȷ����________
A��BaCl2��Na2CO3��NaOH��HCl B��NaOH��BaCl2��Na2CO3��HCl
C��BaCl2��NaOH��Na2CO3��HCl D�� Na2CO3��NaOH��BaCl2��HCl
��2��Ϊ�˼��龫�����Ƿ���SO42-����ȷ�ķ�����____________________________��
II.��������ȡ���������ͼ��ʾ

��1�����չ����У���ʹ�õ��ģ����������⣩ʵ��������______
A���Թ� B�������� C������ǯ D�������� E���ƾ��� F�����ż�
��2��ָ����ȡ��Ĺ������й�ʵ��������ƣ���________��__________��_________
��3����������Ӧ�����ӷ���ʽΪ___________________���ù���������Ҳ������H2O2���������ʵ�����I-ת��ΪI2������Cl2��H2O2�����ʵ���֮��Ϊ__________
��4�����й��ں�����ȡ���˵������ȷ����_________
A��������л��ܼ������Ϻ�ɫ
B�����������ȷų��²�Һ�壬Ȼ���ٴ��¿ڷų��ϲ�Һ��
C��������ʱ���¶ȼƵ�ˮ����Ӧ����Һ�����µ����ܴ�����������ƿ�ĵײ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��D��һ�ִ���ҩ��F��һ�����ϣ����ǵĺϳ�·�����£�
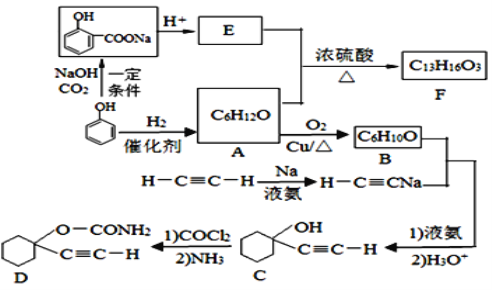
��1��A�Ļ�ѧ������_____________��C�к��������ŵ�����Ϊ_____________��
��2��F�Ľṹ��ʽΪ____________________��A��E����F�ķ�Ӧ����Ϊ______________��
��3��A����B�Ļ�ѧ����ʽΪ__________________________________________________��
��4��д����C�ϳ�D�ĵڶ�����Ӧ�Ļ�ѧ����ʽ��_________________________________��
��5��ͬʱ��������������E��ͬ���칹����_________�֣����������칹����
����FeCl3��Һ������ɫ��Ӧ�� ���ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������¶Ȳ�����ܱ������г���amolX������Y��������Ӧ��2X(g)��Y(s)![]() Z(g)��W(g)������ƽ������ټ���bmolX�������жϲ���ȷ���ǣ� ��
Z(g)��W(g)������ƽ������ټ���bmolX�������жϲ���ȷ���ǣ� ��
A.ƽ�������ƶ�B.X��ת���ʲ���
C.X��ת���ʼ�СD.Y��ת��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com