
���Ľ��й���ҵ����2016��12��11���ڱ����佱������Ŀ֮һΪ���Ҽ�һ�����ҩ�������ᰣ��������з������Ĺؼ��м���G��һ�ֺϳ�·�����£�

��֪����A������ֻ��һ�ֻ�ѧ�������⣻
��TsClΪ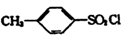 ��
��
��1��A������Ϊ______________��
��2��G�����������ŵ�������______________�� E�Ľṹ��ʽΪ___________________��
��3����B����C�ķ�Ӧ������_____________����F����G�ķ�Ӧ������______________��
��4��PEO����A�����������¼Ӿ۶��ɵ������{���ӣ��üӾ۷�Ӧ�Ļ�ѧ����ʽΪ_______________��
��5����ȡ�������廯����W��D��ͬ���칹�塣W���ܷ���������Ӧ��ˮ�ⷴӦ��������FeCl3��Һ������ɫ��Ӧ��1molW������4molNaOH��Ӧ��W�ĺ˴Ź�������ֻ��4��塣W�Ľṹ��ʽΪ_________________��
��6�����������ϳ�·��, ���������ױ���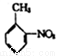 �����Ҵ�Ϊԭ�ϣ����Լ���ѡ��������Ʊ��ڰ���������������
�����Ҵ�Ϊԭ�ϣ����Լ���ѡ��������Ʊ��ڰ���������������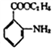 ���ĺϳ�·�ߡ�_________________
���ĺϳ�·�ߡ�_________________
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
����˾���Ƿ�ƺ��ʻ�ķ�Ӧԭ��Ϊ��
3C2H5OH+2CrO3+3H2SO4=3CH3CHO+Cr2(SO4)3+6H2O��ش��������⣺
��1���÷�Ӧ�漰Ԫ���У���̬ԭ�Ӻ���δ�ɶԵ���������Ԫ����Ԫ�����ڱ��е�λ��Ϊ_____________________��
��2������Cr2(SO4)3�����Ԫ�أ��縺�Դ�С�����˳��Ϊ________________������Ԫ�ط��ű�ʾ��
��3��CH3CHO��̼ԭ�ӵĹ���ӻ�������________�֡�
��4����(Mo)����Crͬ��ĵ�������Ԫ�أ���Moԭ�ӵļ۵����Ų�ʽΪ_____________��
��(Mo)�������̵IJ��ֵ��������±���ʾ��
��� | I5/KJ��mol-1 | I6/KJ��mol-1 | I7/KJ��mol-1 | I8/KJ��mol-1 |
A | 6990 | 9220 | 11500 | 18770 |
B | 6702 | 8745 | 15455 | 17820 |
C | 5257 | 6641 | 12125 | 13860 |
A��_____________ (��Ԫ�ط���)��
��5���÷�Ӧÿ����1 mol CH3CHO��C2H5OH������Ӧ���ѦҼ�����ĿΪ__________��
��6����CrCl3��6H2O�ܽ�������ˮ�У���Һ��Cr3+�ԣ�Cr(H2O)5Cl]2+��ʽ����,��Cr(H2O)5Cl]2+�к��ЦҼ�����ĿΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������εķ����ǽ���������ȡ�����������Թ���Ȼ�� ��
A. ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڹ۲���ɫ�仯
B. ��ˮ�ܽ⣬�ú�ɫʯ����ֽ������Һ�����
C. ��NaOHŨ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڹ۲���ɫ�仯
D. ����ǿ����Һ�����ȣ��ٵ����̪��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ�żҿ��и�����һѧ����ĩ��ѧ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
T��ʱ����10L�ݻ�������ܱ������м���1.0molNH2COONH4��������ӦNH2COONH4��s�� CO2��g����2NH3��g�� ��H��0��5min�ﵽƽ��ʱ�����������NH2COONH4��s�������ʵ���Ϊ0.2mol������˵����ȷ����
CO2��g����2NH3��g�� ��H��0��5min�ﵽƽ��ʱ�����������NH2COONH4��s�������ʵ���Ϊ0.2mol������˵����ȷ����
A. 0��5min�ڣ�v��CO2����0.16mol��L��1��min��1
B. ���������������ʱ���÷�Ӧһ���ﵽƽ��״̬
C. ƽ�����С�����ݻ������½���ƽ��ʱ��c��CO2������
D. �����������䣬����ԭ������Ϊ������������ﵽƽ��ʱ��NH2COONH4��ת����С��80%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ�żҿ��и�����һѧ����ĩ��ѧ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A. ����TiCl4Ũ��Һ�Ʊ�TiO2��xH2O��Ti4������x��2��H2O TiO2��xH2O��4H��
TiO2��xH2O��4H��
B. ��NH4��2Fe��SO4��2��Һ�м�������KOH��Һ��
C. ��������������ʴʱ��������Ӧ��O2��4H����4e����2H2O
D. ����KI��Һ��ͨ��O2��4H����2I����O2��2H2O��I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ�ڵڶ����ʼ쿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ζ�������ָ�ζ������б���Һ�������Һ�����ʵ����ʵ���֮�ȡ���0.10mol��L-1NaOH��Һ�ζ�0.10mol��L-1H2C2O4(����)��Һ�ĵζ�������ͼ��ʾ������˵������ȷ���ǣ� ��

A. H2C2O4���ڶ�Ԫ����
B. ����NaOH����Һ�ζ�NaHC2O4��Һ�����÷�̪��ָʾ��
C. ͼ�Тٴ�: c(Na+)+c(H+)=c(HC2O4-)+c(C2O42-)+c(OH-)
D. ͼ�Тڴ���c(Na+)>c(C2O42-)>c(OH-)>c(HC2O4-)>c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��С��鶼��ѧ��������ѧ��ʮ���С�ɣ����ѧ�߶�3��������ѧ�Ծ��������棩 ���ͣ�������
ij�л�����C��H��O����Ԫ����ɣ���ȡ0.1mol��5.6L��������״���£�ǡ�÷�Ӧ��ȫ,���ò���ΪCO2��CO��H2O(��),����ͨ��ʢŨ�����ϴ��ƿ,ϴ��ƿ������������5.4g,��ͨ�����ȵ�����ͭ,����ͭ������������1.6g,��ͨ��װ�м�ʯ�ҵĸ����,�����������8.8g�����л���ķ���ʽ����д�������ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��С��鶼��ѧ��������ѧ��ʮ���С�ɣ����ѧ�߶�3��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧ������ȷ���� ( )
A������Ľṹ��ʽΪ��CH3CH2CH2CH2CH3
B����ȩ�ĵ���ʽΪ��
C���л���CH2=CH��CH2��CH3�ļ���ʽΪ��
D������(CH3COOH)�����ʽΪ��C2H4O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�����и����ڶ���ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
298Kʱ����20mLbmol��L-1����������Һ�е���0.10mol��L-1�Ĵ��ᣬ��Һ��pH�����Ӵ���������ϵ��ͼ��ʾ�������й�������ȷ����

A. a<20
B. C����Һ�У�c(CH3COO-)+c(CH3C00H)=c(Na+)
C. A��B��C��D�ĵ�ˮ�ĵ���̶ȴ�С˳��Ϊ��D>B>C>A
D. ����ĵ���ƽ�ⳣ��Ka��2.0��10-7/(0.la-2)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com