
����Ŀ��ijϡ�����ϡ����Ļ����Һ200mL��ƽ���ֳ����ȷݡ�������һ��������ͭ�ۣ�������ܽ�19.2g (��֪����ֻ����ԭΪNO����)������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ�����з��������������
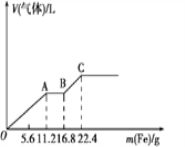
A. OA�β�������NO��AB�εķ�ӦΪFe+2Fe3+=3Fe2+��BC�β�������
B. ԭ�������SO42�����ʵ���Ϊ0.6 mol
C. �ڶ�����Һ����������ΪFeSO4
D. ȡ20mLԭ������ˮϡ����1L����Һ��c(H+)=0.2mol/L
���𰸡�B
��������
�û����Һ��ͭ�۷�Ӧ�����ӷ���ʽΪ��3Cu��8H����2NO3��=3Cu2����2NO����4H2O����֪m(Cu)=19.2g����n(Cu)=0.3mol������òμӷ�Ӧ��n(NO3��)=0.2mol��n(H+)=0.8mol������������Һ��Ӧʱ����������ΪFe3������HNO3����ԭΪNO����OA�εķ�Ӧ��ΪFe��4H����NO3��=Fe3����NO����2H2O���������۵����ӣ�AB�εķ�Ӧ��Ϊ2Fe3����Fe=3Fe2������ʱû���������ɣ�������������ʱ���ֲ������壬��BC�εķ�Ӧ��ΪFe��2H��=Fe2����H2����˵����Һ��ʣ�����H+����֪OA������n( Fe) =0.2mol���ɷ�Ӧ�ڿɵòμӷ�Ӧ��n(NO3��)=0.2mol��n(H+)=0.8mol��BC������n( Fe) =0.1mol����Ӧ�����ĵ�n(H+)=0.2mol���ۺϷ�����֪��ÿһ�ݻ����Һ��n(HNO3)= 0.2mol��n(H2SO4)= 0.4mol��
A�������Һ��ʼ�����۷�Ӧʱ��ϡ�����������ΪFe3��������������ԭΪNO����OA�Σ�����Fe3��Ҳ�н�ǿ�������ԣ������ӵ����ۻ�ԭΪFe2+����AB�Σ�������Һ��ʣ����H+�����������۷�Ӧ����H2����BC�Σ�����A��ȷ��
B��������������֪��ԭ�����Һ��n(H2SO4)= n(SO42-)=2��0.4mol=0.8mol������B����
C�����ݷ�Ӧ�ڢۢܿɵã��ڶ�����Һ�е���������ֻ��FeSO4������C��ȷ��
D��ÿһ�ݻ����Һ(100mL)�к���n(HNO3)= 0.2mol��n(H2SO4)= 0.4mol����c(H+)=10 mol/L��ȡ20mL��ˮϡ����1Lʱ���������ʵ����ʵ������䣬�ɵ�0.02L��10mol/L=1L��c(H+)��c(H+)=0.2mol/L������D��ȷ�������ΪB��


 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ڽ����ƴ��������Ҵ���ϼ�������ʹ����ԭ�ɴ�����ӦΪRCOOR�䣫4[H]![]() RCH2OH��R��OH��������C5H11COOC6H13��������������ԭ�����õ��IJ�����
RCH2OH��R��OH��������C5H11COOC6H13��������������ԭ�����õ��IJ�����
A. C6H13OH��C5H11OH B. C5H11OH C. C6H13OH D. C11H23OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����Ӿ�������ݴ�С�Ƚϲ���ȷ����(����)
A. �����ܣ�NaF>NaCl>NaBr
B. Ӳ�ȣ�MgO>CaO>BaO
C. �۵㣺NaF>MgF2>AlF3
D. �����ӵ���λ����CsCl>NaCl>CaF2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijװ�к�ɫ��Һ���Թܣ�����ʱ��Һ��ɫ��dz����ԭ��Һ������
�ٵ��з�̪�İ�ˮ��Һ �ڵ��з�̪������������Һ
������SO2��Ʒ����Һ �ܵ��з�̪�ı�������������Һ
�ݷ�̪��Һ�еμ�����NaClO��Һ
A. �٢ܢ� B. �٢� C. �ۢܢ� D. �٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������������Һ��Ӧ���ǣ� ��
A.NO2B.Fe2O3C.SO3D.NaHCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƾ���(Na2S2O3��5H2O)��������������ɫ��б���塣��������ˮ���������Ҵ������н�ǿ�Ļ�ԭ�ԣ���Ӧ��������ȹ�ҵ�С��ش��������⣺
(1)���������£�S2O32-��������������ԭ��Ӧ����SO2����д��Na2S2O3�����ᷴӦ�����ӷ���ʽ��_________________________________________��
(2)�������Ʒ��Ʊ�Na2S2O3��5H2O�����������£�

��Na2S2O3��5H2O�Ʊ�ԭ��Ϊ_________________________________________���û�ѧ����ʽ��ʾ����
��Na2S2O3��5H2O��Ʒ�п��ܺ���Na2SO3��Na2SO4���ʣ�����鲽��Ϊ��ȡ������Ʒ���ϡ��Һ���μ������Ȼ�����Һ���а�ɫ�������ɣ����ˣ���������ˮϴ�ӳ�����Ȼ��������м�������______________�����Լ����ƣ�����____________����������֤����Ʒ�к���Na2SO3��Na2SO4��
�۴�Ʒ��Na2S2O3��5H2O�����������IJⶨ��ȡ5g��Ʒ����250 mL����Һ���á���ȡ25.00 mL 0.0100 mol�� L-1K2Cr2O7��Һ����ƿ�У�Ȼ���������ữ��KI��Һ���ữ�ͼ��ε�����Һ�����������Ƶ�Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ25.00 mL���ζ��յ��������____________________����д��K2Cr2O7��Һ��������ữ��KI��Һ��Ӧ�����ӷ���ʽ��____________________________________________����Ʒ��Na2S2O3��5H2O����������Ϊ_________������֪I2+2S2O32-=2I-+S4O62-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں��д�����H����Ba2����Cl������Һ�У������ܴ�������������ǣ� ��
A.OH-B.Mg2��C.Ag��D.CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ������лᵼ�½��ƫ�ߵ��� �� ��
�� ����ϡH2SO4ʱ��ϴ����ȡŨH2SO4�����Ͳ������ϴ��Һת�Ƶ�����ƿ��
�� ������Һʱ��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�ж���
�� ����к͵ζ�ʱ��ʢװ��Һ�ĵζ���������ˮϴ����ֱ�Ӽ����Һ
�� ����к͵ζ�ʱ���ζ�ǰû���ų��ζ��ܼ��촦������
�� ������Һʱ��ת��ǰ������ƿ�к�����������ˮ
�� ������Һʱ������ҡ�Ⱥ���Һ����ڿ̶���
�� ������Һʱ������ʱ�����ӿ̶���
A. �٢ڢۢܢ� B. �٢ڢܢޢ� C. �٢ڢݢ� D. �٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£��� aLHCl��ȫ���� 1 L ˮ�� ( ˮ���ܶȽ���Ϊ 1 g / mL) ����Һ���ܶ�Ϊ d g/cm 3����Һ�����ΪV mL�����ʵ���������Ϊ�أ����ʵ����ʵ���Ũ��Ϊc mol/L �����������в���ȷ����(�� ��)
A. �� ��![]() ��100%
��100%
B. c =![]() mol/L
mol/L
C. ������Һ�к���HCl����
D. ��������Һ���ټ���V mL ˮ��������Һ�������������� 0.5��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com