
��֪���������[(NH4)2SO4��FeSO4��6H2O] ���׳�Ī���Σ�������ˮ����100�桫110��ʱ�ֽ⡣Ϊ̽���仯ѧ���ʣ��ס�����ͬѧ���������ʵ�顣
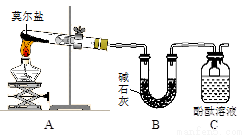
��̽��Ī���ξ������ʱ�ķֽ���
��ͬѧ�������ͼ��ʾ��װ�ý���ʵ�飬װ��C�пɹ۲쵽��������____________________���ɴ˿�֪�ֽ��������_______________��
��ͬѧ��ΪĪ���ξ���ֽ�IJ����л����ܺ���SO3(g)��SO2(g)��N2(g)��Ϊ��֤����Ĵ��ڣ�������װ�ý���ʵ�顣
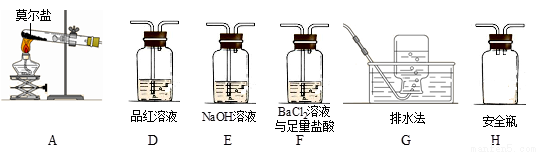
����ͬѧ��ʵ���У�װ���������ӵĺ���˳��Ϊ��A��H����____������____������____����G��
��֤������SO3��ʵ��������______________����ȫƿH��������____��
��Ϊ�ⶨ��������林��ȣ���ȡm gĪ������Ʒ�����500 mL��Һ���ס�����λͬѧ�������������ʵ�鷽����������ȡ25.00 mL��Ʒ��Һ��0.1000 mol��L��1������K2Cr2O7 ��Һ�����ν��еζ����ҷ�������ͨ��NH4+�ⶨ��ʵ�����װ������ͼ��ʾ��ȡ25.00 mL��Ʒ��Һ���и�ʵ�顣
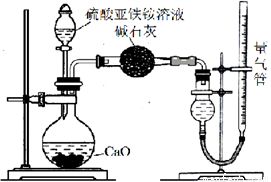
��ش�
��1�������е����ӷ���ʽΪ��________________________��
��2���ҷ�����������������Լ���________
a��ˮ b������NaHCO3��Һ c��CCl4
��3���ҷ������ռ������岢�ָ������£�����ǰӦ���еIJ�����________________��
��4�������NH3ΪV L(������Ϊ��״����)������������林���Ϊ_____���г�����ʽ����
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�������и�����Ӧ���¿����壩���ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
I. Ϊ�Ƚ�Cl2��Fe3+��SO2�������ԣ�����ͼ��ʾװ�ý���ʵ�飬��������£�

��.���ɼ�K1��K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K3��K4��
��.����a���μ�һ������Ũ���ᣬ��A���ȡ�
��.��B�е���Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��.����b��ʹԼ2mL����Һ����D�Թ��У��������е����ӡ�
��.���ɼ�K3������c������70%�����ᣬһ��ʱ���н����ɼ�K3��
��.�����Թ�D���ظ����̢ܣ�����B��Һ�е����ӡ�
��1�����н������ҺΪ__________��
��2����A�������������36.5%�ܶ�Ϊ1.2g/mL����100mLʱ���䷴Ӧת�Ƶĵ�����ĿΪ______��
��3�����̢��м���B��Һ���Ƿ�����������ӵIJ�����___________��
��4���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬���ǵļ����һ���ܹ�֤�������Ե���____ͬѧ����������˳��Ϊ____________��
���̢� B��Һ�к��е����� | ���̢� B��Һ �к��е����� | |
�� | ��Fe3+��Fe2+ | ��SO42�� |
�� | ����Fe3+����Fe2+ | ��SO42�� |
�� | ��Fe3+��Fe2+ | ��Fe2+ |
II. NaNO2����Ҫ�ķ�������+3�۵ĵ�����������Ӧ�������Ի����в��ȶ�����5mol/Lˮ��ҺpHΪ9��ij��ѧ��ȤС���������ͼ��ʾװ���Ʊ��������ơ�������Ǣٹرյ��ɼУ���A�з�Һ©���������μ�һ����Ũ���ᣬ���ȣ���һ��ʱ���ֹͣ���ȡ��ش��������⣺
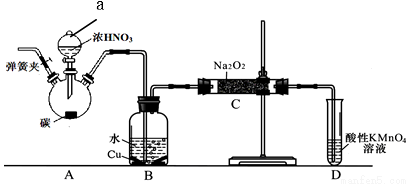
��1��B�й۲����Ҫ������__________��Dװ�õ�������____________��
��2������C�в������������ƵIJ�����_______����Ӧ��Ӧ����ʽΪ___________��
��3��������C�в����������ƺ������١�a. ��ͬѧ��ΪC�в��ﲻ�����������ƣ������������ʡ�Ϊ�ų����ţ�����B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ���_______��д���ƣ���b. ��ͬѧ��Ϊ���������������⣬�������������뷴Ӧ���²�Ʒ������������ʵ�������ǰӦ����һ���������ò�����___��
��4���������������HNO2��Ka��ֵΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ��ƶ���
�й����ʵ�ת����ϵ����ͼ��ʾ(������������������ȥ)����֪A��������Ԫ����ɣ���Ħ������Ϊ32 g��mol��1������������ƽ�����B��һ�ֺ���ɫ���壬C�ǿ����к�����ߵĵ��ʣ�D�������Һ�壬LΪ����ɫ���壬I��һ�ֽ�����K����ɫ��ӦΪ��ɫ��
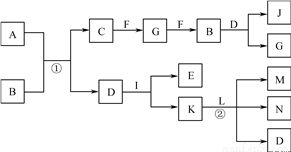
(1) J�Ļ�ѧʽΪ______________��
(2) C�ĵ���ʽΪ______________��
(3) д����Ӧ�ٵĻ�ѧ����ʽ��______________________________________________��
(4) д����Ӧ�ڵ����ӷ���ʽ��______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
����ɫ������Һ���ܴ��������һ��������
A. Cu2����K����NO3-��Cl�� B. Na����K����OH����Cl��
C. Mg2����Na����OH����SO42�� D. Ba2����H����CO32����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
ʯīϩ�������ȴҲ�����Ӳ�IJ��ϣ�����������δ���ij�������������ʯīϩ��Ԫ����
A. ̼ B. �� C. �� D. ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л����������ȷ����
A. ��ϩ��������ϩ�ͱ������о�����̼̼˫��
B. ������֬������ʹ����KMnO4��Һ��ɫ
C. �ȱ�����������ԭ�Ӷ�����ͬһƽ��
D. �ױ������ϵ�һ����ԭ�ӱ���C3H6Clȡ�����γɵ�ͬ���칹����9��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ������
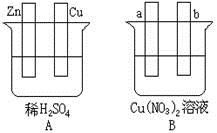
��1����Aͼ�У�ʹͭƬ��ðH2���ݡ�����Ա�Ҫ���ӣ������Ӻ��װ�ý� ���缫��Ӧʽ��п���� ��ͭ���� ��
��2����Bͼ�У�a��b��Ϊ���Ե缫����a������ͭ����b���� �����Ա�Ҫ�����Ӻ�װ�ý� ���缫��Ӧʽ��a���� b���� ������һ��ʱ���ֹͣ��Ӧ��������Һ����Һ��H+Ũ�� �����ߡ����͡����䣩������һ������ ����Һ�ָܻ�������ǰ��ȫһ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ҵ����ȡCuCl2�������������£�

�����±����ݣ��ش��������⣺
�� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
�� ��ҺA�м���NaClO��Ŀ���� ��
�˷�Ӧ���ӷ���ʽΪ ��
�� ����ҺB�м���CuO�������� ��
�� ����a��Ŀ���� ��
�� ��Cu(OH)2��������ʹCu(OH)2ת��ΪCuCl2�����ö�������͵������ɵ�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£������������ڸ���������һ���ܴ����������
A. pH��1����Һ�У�K����Na����NO3����CH3COO��
B. Na2S��Һ�У�K����Na����NO3����Ag��
C. KCl��Һ�У�Na����Fe3����SO42����SCN��
D. c��OH������1��10��13mol��L��1����Һ�У�K����Mg2����Cl����NO3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com