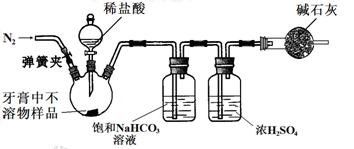
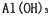��2012?������ģ�⣩��������Ħ������һЩ������ˮ��������ɣ����õ�Ħ������Ҫ�У�CaCO
3��Al��OH��
3��SiO
2?nH
2O��Ca
3��PO
4��
2��������ʾ��Ca
3��PO
4��
2���²��ֽ⣻Ca
3��PO
4��
2��CaHPO
4������ˮ��Ca��H
2PO
4��
2����ˮ��Ca
3��PO
4��
2+4H
+=3Ca
2++2H
2PO
4-��
��1��Ϊ�ⶨij���������Ժ�Ħ��������Ҫ�ɷ֣���ͬѧ��Ʋ��������ʵ�飺
��ȡһС��������һ��������ˮ��ֽ������ˣ���pH��ֽ�ⶨ��Һ����Եķ�����
�ø���ྻ�IJ�����պ��Һ������pH��ֽ�ϣ��������ɫ���ȽϺ�
�ø���ྻ�IJ�����պ��Һ������pH��ֽ�ϣ��������ɫ���ȽϺ�
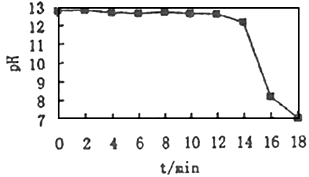
������Ӧ��������������pH�ӽ�8��
����������ò������м�������ϡ���ᣬ�õ�������Һ��������������������������ӷ���ʽ��
CaCO3+2H+=Ca2++CO2��+H2O
CaCO3+2H+=Ca2++CO2��+H2O
����������ò������м���NaOH��Һ�����ⶨ��������������������仯��
��ȡ���м�ϡ�����������Һ���������NaOH��Һ���۲쵽�����г������ɣ����ɸó��������ӷ���ʽ��
2H2PO4-+3Ca2++4OH-=Ca3��PO4��2+4H2O
2H2PO4-+3Ca2++4OH-=Ca3��PO4��2+4H2O
����������ʵ�飬Ħ������һ�����е�������
CaCO3��Ca3��PO4��2
CaCO3��Ca3��PO4��2
��
��2����ͬѧ������װ�ã��Ѽ��������ԣ��г�װ���ԣ�ͨ����CO
2���������������������CaCO
3�����

�ٵμ�ϡ����֮ǰ�IJ��ֲ����������ɼУ�ͨ��N
2һ��ʱ�䣻�ٽ�ʢ�м�ʯ�ҵĸ��������������װ���ϡ�������˳����в�����ԭ����
�����ʯ������װ����ԭ�п����е�CO2
�����ʯ������װ����ԭ�п����е�CO2
��
��������װ���ظ�����ʵ�飬������Ʒ��CaCO
3�ĺ�����ƫ�ߣ���ʵ������еIJ���û��ʧ�������ܵ�ԭ����
������еļ�ʯ�����տ����е�CO2��H2O
������еļ�ʯ�����տ����е�CO2��H2O
��
NaHCO3��Һ��HClʱ������CO2
NaHCO3��Һ��HClʱ������CO2
��
�����ʵ�鲻�������κ��Լ�����������ѡ�����������������Ʒ�ⶨĦ������CaCO
3�����ķ�����
������������Ʒ�ڸ�������ȫ�ֽ�ǰ�������
������������Ʒ�ڸ�������ȫ�ֽ�ǰ�������
������������������