
��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20 mL 0.1 mol/L CH3COOH��Һ����μ���0.1 mol/L NaOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)������˵���в���ȷ���ǡ��������������������������������� ���� ��
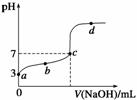
A��a���ʾ��Һ��c(CH3COO��)��10-3 mol/L
B��b���ʾ����Һ��c(CH3COO��)��c(Na��)
C��c���ʾCH3COOH��NaOHǡ�÷�Ӧ��ȫ
D��b��d���ʾ����Һ��![]() ������K
������K

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
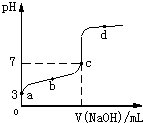 ��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL0.1mol/LCH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL0.1mol/LCH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?���ݶ�ģ����֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1 NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
��2009?���ݶ�ģ����֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1 NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1 NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1 NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
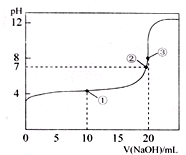 ��2010?������ģ�⣩��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶��£���0.1000mol?L-1NaOH��Һ�ζ�20.00mL 0.1000mol?L-1CH3COOH��Һ���õζ�������ͼ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
��2010?������ģ�⣩��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶��£���0.1000mol?L-1NaOH��Һ�ζ�20.00mL 0.1000mol?L-1CH3COOH��Һ���õζ�������ͼ�������¶ȱ仯��������˵���в���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20 mL 0.1 mol��L��1 CH3COOH��Һ����μ���0.1 mol��L��1 NaOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)������˵���в���ȷ���ǣ� ��

A��a��ʱ��CH3COOH�ĵ������1%
B��b��ʱ����Һ��c(CH3COO��)��c(Na��)
C��c���ʾCH3COOH��NaOHǡ�÷�Ӧ��ȫ
D��b��d���ʾ����Һ�� ������K
������K
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com