
����Ŀ�������£���0.1 mol��L��1 NaOH��Һ�ζ�10 mL 0.1 mol��L��1 H2X��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������˵������ȷ����

A.ˮ���������c(OH��)��D�㣾B��
B.C����ڹ�ϵʽ��c(Na��)��c(HX��)��c(X2��)��c(H��)
C.B�㣺c(HX��)��c(H��)��c(X2��)��c(H2X)
D.A����Һ�м�������ˮ��![]() ����
����
���𰸡�B
��������
A��B�㷴Ӧ������ΪNaHA��HA-�ĵ���̶ȴ�����ˮ��̶ȣ���Һ�����ԣ�������������ˮ�ĵ��룬��D�����20mL����������Һ������ǡ�÷�Ӧ����Na2A��A2-ˮ��ٽ���ˮ�ĵ��룬����ˮ�����c(OH-)��B�㣼D�㣬��A��ȷ��
B����Һ���ڵ���غ�c(Na+)+c(H+)=c(HX-)+2c(X-)+c(OH-)����c(Na+)=c(HX-)+c(X2-)+c(OH-)-c(H+)����B����
C��B��ʱ������10mLNaOH��Һ����Ӧ������ΪNaHA����ʱ��Һ��pHС��7��˵��HA-�ĵ���̶ȴ�����ˮ��̶ȣ���c(A2-)��c(H2A)�����������ӻ�����ˮ�ĵ��룬��c(H+)��c(A2-)����Һ������Ũ�ȴ�СΪ��c(HA-)��c(H+)��c(A2-)��c(H2A)����C��ȷ��
D������ͼ���֪��0.1mol/L��H2A��Һ��pH����1��˵��H2AΪ���ᣬ��A����Һ�м�������ˮ����Һ�������ӡ�H2A��Ũ�ȼ�С������ˮ�����ӻ����䣬������������Ũ����������![]() �ı�ֵ����D��ȷ��
�ı�ֵ����D��ȷ��
�ʴ�ΪB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������һ�ֿ��ס���ʹ�����ȵij���ҩ���䲿�ֺϳ�·�����¡�

��ش�
��1���ҵĽṹ��ʽ��________________��
��2��������к��������ŵ�������_____________________��
��3���� ![]() �ҵķ�Ӧ������____________��
�ҵķ�Ӧ������____________��
��4������������Dz���Ҵ�Ʒ���ƹ����в�����һ�����ʡ���������Ҵ��������������»����ɲ�����������÷�Ӧ�Ļ�ѧ����ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ұ߷�Ӧ��ͼ��գ���֪��Ӧ���ǹ�ҵ������������D�ķ�Ӧ����Ӧ����ʵ���Ҽ���������E�ķ�Ӧ����ҵ����ȡƯ�۵ķ�ӦҲ�����С�

��1������L��_____ ��
��2��������B��____��
��3��ͼ�г���Ӧ�����⣬�����������ڹ�ҵ�����ķ�Ӧ����________��_______������ţ���
��д�����ǵĻ�ѧ��Ӧ����ʽ��____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
����ȡ25.00mL����Һ�������л����μ�����Һ15.00mL�����ռ���224mL����״��������
����ȡ15.00mL����Һ�������л����μӼ���Һ25.00mL�����ռ���112mL����״��������
��1���жϣ�����___��Һ�������ʵ���Ũ��Ϊ___mol/L��
��2��ʵ�������������Ӧ�����ӷ���ʽΪ___��
��.��51.2gCu��ȫ��������Ũ�����У��ռ��������������NO��N2O4��NO2���Ļ�����Щ����ǡ���ܱ�500mL2.0mol/LNaOH��Һ��ȫ���գ����ɺ�NaNO3��NaNO2������Һ��������NaNO3�����ʵ���___��Ҫ���м�������벽�裩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��Ϊ������Ԫ�أ���������Ϊ����Ԫ�أ�һ��Ϊ�ǽ���Ԫ�أ���ԭ�Ӱ뾶�ֱ�Ϊ
X | Y | Z | |
ԭ�Ӱ뾶/nm | 0.154 | 0.130 | 0.071 |
X��Y����ͬһ���ڣ�����Ԫ���γɵļ����Ӿ�����ͬ�ĵ��Ӳ�ṹ������˵����ȷ����
A. ԭ��������������Z>X>Y
B. ����Ԫ�ؿ���Ϊͬ����Ԫ��
C. ԭ��������Y>X>Z
D. ���Ӱ뾶��X>Y>Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ƽ�����ھ��������ƣ����ĺϳ�·�����£�

��1��д��C13H9NO4S�����к��������ŵ�����__________��
��2��A������������Է���������54��д��A�Ľṹ��ʽ_______________��
��3����Ӧ�١���������ȡ����Ӧ����____��ѡ����ţ���
д����Ӧ�ߵĻ�ѧ����ʽ________________��
��4����������Ʒ�Ӧ�ݺ͢ߵ�Ŀ����_____________________��
��5������C��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹����____�֡�
��6����֪�������ϵ��Ȼ�Ϊ��λ��λ������ ��д����
��д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�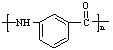 �ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�![]() ��_____________
��_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̽����Һ����Զ�![]() ��Һ�����ƺͱ����Ӱ�졣10mL
��Һ�����ƺͱ����Ӱ�졣10mL![]() ��10mL
��10mL![]() ��Һ�С�
��Һ�С�
I.![]() ��Һ������
��Һ������
��![]() �ֱ�����10mL����ˮ��10mL
�ֱ�����10mL����ˮ��10mL
��Һ��� | �ܼ� | ��Һ��״ | |
�� | ����ˮ | dz��ɫ������Һ�� | �� |
�� |
| ��ɫ������Һ�� | �� |
�� |
| ��ɫ������Һ | �� |
(1)��ƽ���ƶ�ԭ�����͢������Ե���Ҫԭ����________________
(2)�ڡ��۱�췢����Ӧ�����ӷ���ʽ��_______________________
(3)��ͬѧ��Ϊ����һ����+3���������ü������������Һ��,�۲쵽��·:������KSCN�Ģ��е����Լ�a,��Һ���,˵�����ƶ���ȷ���Լ�a��____________
II ![]() ��Һ�ı���
��Һ�ı���
��ʵ��I�����Ƶ�������Һ�ֱ��ڿ����з���24Сʱ��,��¼���¡�
��Һ��� | ��Һ��״ | |
�� | ��ɫ���� | �� |
�� | ��ɫ��Һ | �μ�5�� |
�� | ��ɫ��Һ | �μ�5�� |
���������ۣ��٢�˵�����Լ���ʱ,![]() ��Һ���ױ��ʣ��ڢ�˵������
��Һ���ױ��ʣ��ڢ�˵������
���������ϣ���һ��pH��Χ�ڣ�+2�����Ļ�ԭ�������Լ�������ǿ,�����������������Ե���ǿ����ǿ��
������ʵ�飩����ͼ��ʾװ�����ʵ��(�μ��Լ�ʱ��Һ�������Һ�����Ա仯�ɺ���)�����ҳس����ȶ�ͨ������,��������ʾ���ȶ���:

I ����صμ�Ũ������![]() �ӽ�
�ӽ�![]() ������û�����Ա仯
������û�����Ա仯
II ���ҳصμӵ���Ũ���ᣬ������������
(4)��ȫ���������ۣ�:�ڢ�˵��_______________
(5)ii���ҳصĵ缫��Ӧ����ʽ��____________________
(6)����ʵ���ƶϣ��۱Ȣ���![]() ���ױ��ʵ�ԭ����___________________
���ױ��ʵ�ԭ����___________________
(7)������ԭ����װ�����½���ʵ��֤ʵ����![]() ���ױ��ʵ�ԭ��ʵ�鷽����Ԥ��������:���ҳس����ȶ�ͨ������,��������ʾ���ȶ���____________
���ױ��ʵ�ԭ��ʵ�鷽����Ԥ��������:���ҳس����ȶ�ͨ������,��������ʾ���ȶ���____________
(8)����ʵ��,���Ʋ�����![]() ��Һ����ѷ���_________________��
��Һ����ѷ���_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.������8�־��壬����Żش��������⣺
A��ˮ�� B�������� C������ D����̬� E���Ȼ�� F���� G�����ʯ
��1������ԭ�Ӿ���Ļ�������___���������Ӿ������___��������ѧ���ķ��Ӿ�����____��
��2���ɼ��Է��ӹ��ɵľ�����___�����й��ۼ������Ӿ�����___�����ڷ��Ӿ���ĵ�����____��
��3�������ڴ��ڻ�ѧ�����������ۻ�ʱ����ѧ���������仯����___�������ۻ�����˷����ۼ�����____��
��.���мס��ҡ����������־���(��ͼ��ʾ)������֪��������A��B�����Ӹ�����Ϊ___���Ҿ���Ļ�ѧʽΪ___��������Ļ�ѧʽΪ____��������Ļ�ѧʽΪ____��
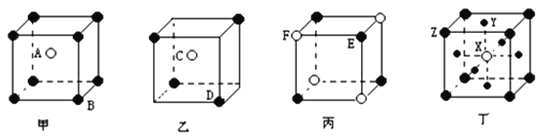
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���һ����� 0.1mol/L ������Һ����μ����Ũ�ȵ� NaOH ��Һ����Һ��pOH �� pH �ı仯��ϵ��ͼ��ʾ������ȷ����( )

A. M ����ʾ��Һ��������ǿ�� Q ��
B. N ����ʾ��Һ�� c(Na+)>c(CH3COO-)
C. Q ����ʾ����Һ pH һ������ 7
D. Q ����� NaOH ��Һ��������ڴ�����Һ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com