
ijͬѧ����������Ϊ98�����ܶ�Ϊ1.84 g��cm3��Ũ���ᣬ����100 mL 2 mol��L H2SO4��Һ���������йص�ʵ�顣�Իش��������⣺
��1����������Ũ����������
��2��������������ѡ��ʵ������Ҫ������ (�����)��
| A��10 mL��Ͳ | B��20 mL��Ͳ | C��100 mL�ձ� | D��100 mL����ƿ |
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��NaHCO3��KHCO3��ɵĻ�������ʵ�飬����������ݣ���������ʵ���Ũ����ȣ������з�����������ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
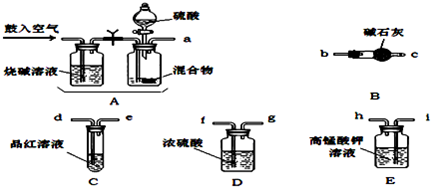
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
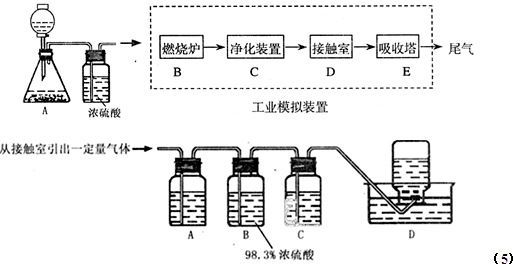
| ||
| ||
| ||
| ||
| ѹǿ/Mpa ת���� �¶�/�� |
0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��.��֪A��BΪ���ʣ�DΪ������������Ϊ�л������A��B��һ�������¿ɻ�������D��Z���з�����ζ��X��Y��Ϊ�����г����л��X�ڴ��������¿���B��Ӧ����C����ͬ�����£�C�������ܶ���H2�ܶȵ�22����C��̼Ԫ�ص���������Ϊ54.5%����Ԫ�ص���������Ϊ9.1%������Ϊ��Ԫ�ء���д����
�ٷ�ӦX+Y![]() Z+D�Ļ�ѧ����ʽ��_________________________________________;
Z+D�Ļ�ѧ����ʽ��_________________________________________;
��A+C![]() X�ķ�Ӧ���ͣ�__________________________________________________;
X�ķ�Ӧ���ͣ�__________________________________________________;
��Y��һ��ͬ���칹��Ľṹ��ʽ��_______________________��
��.��֪A��B��C��DΪ���ʣ�X��Y��ZΪ�������
��1����B��DΪͬһ�����ʣ���д����ӦX+Y![]() Z+D�Ļ�ѧ����ʽ��д��һ�����ɣ���_______________________________________________��
Z+D�Ļ�ѧ����ʽ��д��һ�����ɣ���_______________________________________________��
��2����C��DΪͬһ�����ʣ�
�ٻ�����Z��������Ԫ��Ϊ______________����A��B��C��ʾ����
�ڷ�ӦX+Y![]() Z+D�Ƿ�Ϊ������ԭ��Ӧ����˵�����ɡ�
Z+D�Ƿ�Ϊ������ԭ��Ӧ����˵�����ɡ�
��ijͬѧ��Ϊ��CԪ���ڻ�����X��Y�г��ֵĻ��ϼۣ��ض�����һ�������г����ۣ�����һ�������гʸ��ۡ������жϸý����Ƿ���ȷ�������йصĻ�ѧ����ʽ֤����Ľ��ۡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������A��B��C��D��X��Y��Z֮��ת����ϵ����ͼ��ʾ��
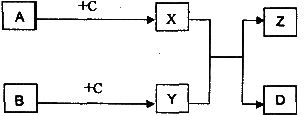
I����֪A��BΪ���ʣ�DΪ������������Ϊ�л������A��B��һ�������¿ɻ�������D��Z���з�����ζ��X��Y��Ϊ�����г����л��X�ڴ��������¿���B��Ӧ����C����ͬ�����£�C�������ܶ���H2�ܶȵ�22����C��̼Ԫ�ص���������Ϊ54.5������Ԫ�ص���������Ϊ9.1��������Ϊ��Ԫ�ء���д����
�ٷ�ӦX+Y��Z+D�Ļ�ѧ����ʽ��______________________
��A+C��X�ķ�Ӧ���ͣ�_______________________
��Y��һ��ͬ���칹��Ľṹ��ʽ��_______________________
����֪A��B��C��DΪ���ʣ�X��Y��ZΪ�������
(1)��B��DΪͬһ�����ʣ���д����ӦX+Y��Z+D�Ļ�ѧ����ʽ(д��һ������)��
___________________________________________
(2)��C��DΪͬһ�����ʣ�
�ٻ�����Z��������Ԫ��Ϊ_________(��A��B��C��ʾ)��
�ڷ�ӦX+Y��Z+D�Ƿ�Ϊ������ԭ��Ӧ?��˵�����ɡ�
��ijͬѧ��Ϊ��CԪ���ڻ�����X��Y�г��ֵĻ��ϼۣ��ض�����һ�������г����ۣ�����һ�������гʸ��ۡ������жϸý����Ƿ���ȷ�������йصĻ�ѧ����ʽ֤����Ľ��ۡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com