
����ʵ��������������ȷ����( )
ѡ�� | ʵ�� | ���� |
A | ���ȷ��������е�С�� | �����ۻ��ɹ�����С��ȼ��ʱ������Ϊ��ɫ��ȼ�պ����ɵ���ɫ���� |
B | �ھƾ����ϼ������� | �����ۻ���ʧȥ�����ۻ������������䣬������һ��Ĥ���� |
C | ��FeCl2��Һ�е���NaOH��Һ | �����ɰ�ɫ�����������ܿ��Ϊ����ɫ������Ϊ���ɫ |
D | �ڿ����о��õ���������NaOH��Һ�� | ���̲���������ɫ���ݣ�������ϸ���������� |
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ12���¿���ѧ�Ծ��������棩 ���ͣ������
Fe(OH)2�ܲ��ȶ���¶���ڿ��������ױ�������Fe(OH)2�������Ļ�ѧ����Ϊ�� ��Ϊ�˻�ð�ɫ��Fe(OH)2�����������ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
(1)Ϊ��ֹFeSO4��Һ�к���Fe3+����������Һ�м���________________��
(2)��ȥ����ˮ���ܽ��O2������ �ķ�����
(3)���ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ�� ����FeSO4 ��ҺҺ���£��ټ���NaOH��Һ������������������ ��
����FeSO4 ��ҺҺ���£��ټ���NaOH��Һ������������������ ��
(4)����FeSO4��Һ���Ƿ���Fe3+���ڵ�����Լ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��������һ�е���У�߶�12��������ѧ���������棩 ���ͣ�ѡ����
��ˮ�м�������NaHSO4�����¶Ȳ���ʱ����Һ�� ( )
A��c(H��)/c(OH��)���� B��c(H��)��С
C��ˮ��c(H��)��c(OH��)�ij˻����� D��c(OH��)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и�һ����ĩ��ѧ���������棩 ���ͣ�ѡ����
��һ��������Mg��Al�Ļ����Ͷ��500 mLϡ�����У�����ȫ���ܽⲢ�������塣����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ����ͼ��ʾ��������˵����ȷ����( )

A��Mg��Al��������Ϊ8 g
B��ԭϡ������Һ�����ʵ���Ũ��Ϊ5 mol��L��1
C�����ɵ�H2�ڱ�״���µ����Ϊ11.2 L
D��NaOH��Һ�����ʵ���Ũ��Ϊ5 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и�һ����ĩ��ѧ���������棩 ���ͣ�ѡ����
�ɶ��������Ƹߴ�����������£������ж��д������( )
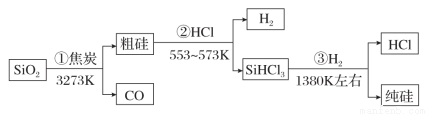
A���٢ڢ۾�����������ԭ��Ӧ
B��H2��HCl����ѭ������
C��SiO2��һ�ּ�Ӳ���۵Ĺ���
D��SiHCl3Ħ������Ϊ135.5 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ����������������и�һ����ĩ��ѧ���������棩 ���ͣ�ѡ����
�������ӷ���ʽ��д��ȷ����( )
A������ͨ��ˮ�У�Cl2��H2O=2H����Cl����ClO��
B������ϡ���ᷴӦ��Fe��2H��=Fe2����H2��
C��̼�������ᷴӦ��CaCO3��2H��=Ca2����CO2����H2O
D����������������������Һ��Ӧ��Al(OH)3��OH��=AlO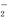 ��2H2O
��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�������¿��廯ѧ�Ծ��������棩 ���ͣ������
����KMnO4��H2O2���������������ҽ���г�����������������H2O2��������Ư�ף��ǻ�ѧʵ������ر�����Ҫ�����Լ������������ɵ����տ��û�ԭ�ԵIJ���(H2C2O4 )ȥ����Fe(NO3)3Ҳ����Ҫ�����Լ��������Ƕ����������������ʵ�̽����
(1)ijͬѧ�����ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬д���÷�Ӧ�����ӷ���ʽ____________________,���������뻹ԭ��������ʵ���֮��_________.
(2)ȡ300 mL 0.2 mol/L��KI��Һ��һ����������KMnO4��Һǡ�÷�Ӧ�����ɵ����ʵ�����I2��KIO3����ת�Ƶ��ӵ����ʵ�������________mol��
(3)�ⶨKMnO4��Ʒ�Ĵ��ȿ��ñ�Na2S2O3��Һ���еζ���ȡ0.474 g KMnO4��Ʒ�ܽ��ữ����0.100 mol/L��Na2S2O3��Һ���еζ�����Na2S2O3��ҺӦʢװ��________(���ʽ����ʽ��)�ζ����С�ʵ���У��ζ����յ�ʱ����Na2S2O3��Һ12.00 mL�������Ʒ��KMnO4�����ʵ�����________��(�й����ӷ���ʽΪ��8MnO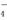 ��5S2O
��5S2O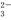 ��14H����8Mn2����10SO
��14H����8Mn2����10SO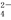 ��7H2O��
��7H2O��
(4)Mg��H2O2���Ե�ز��ú�ˮ���������Һ(����һ������ϡ����)���õ�ص������ķ�ӦʽΪ_______________���ŵ�ʱ����������Һ��PH________��
(5)��Fe(NO3)3��Һ�м���Na2SO3��Һ����Һ�����ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ����Һ�ȱ�Ϊdz��ɫ��ԭ����_________________���ֱ�Ϊ�ػ�ɫ�����ӷ���ʽ��_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�������¿�����ѧ�Ծ��������棩 ���ͣ�ѡ����
������Ԫ��W��X��Y��Z��ԭ��������������W�ĵ����ǿ�������������������壬W��Y����������֮��ΪX������������2����X��Y��Z�����ӵĵ��Ӳ�ṹ��ͬ��Z�����������������ڲ������������˵����ȷ����( )
A. ����Z���ú�ˮ��ԭ�ϻ��
B. W�ļ���̬�⻯���Y�ļ���̬�⻯���ȶ�
C. ԭ�Ӱ뾶�ɴ�С��˳��Z��Y��X��W
D. WX��ZX�еĻ�ѧ��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
��֪�����������ݣ�7.2��10-4��4.6��10-4��4.9��10-10�ֱ��������йص�������ĵ��볣��������֪���з�Ӧ���Է�����
��NaCN��HNO2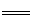 HCN��NaNO2
HCN��NaNO2
��NaCN��HF HCN��NaF
HCN��NaF
��NaNO2��HF HNO2��NaF
HNO2��NaF
�ɴ˿��ж�������������ȷ���� ( )
A��K(HCN)=7.2��10��4
B��K(HCN)<K(HF)<K(HNO2)
C��ͬŨ�ȵ���������Һ��pH(NaNO2)<pH(NaF)<pH(NaCN)
D������֪���ԣ�CH3COOH<HNO2������Է�����ӦHF+CH3COONa NaF+CH3COOH
NaF+CH3COOH
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com