
��13�֣�����̼�����ƺ�̼���ƹ������Ϊ�˲ⶨ�������̼���Ƶİٷֺ�������������װ�ã�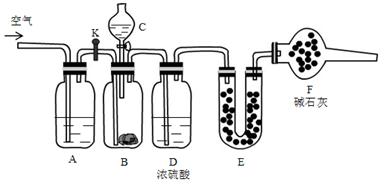
ʵ�鲽�裺
�ټ��װ��������
�ڽ�ҩƷװ�ã�����B��װ�� 9.5g��Ʒ��Eװ��ҩƷ����������56.0g
�۹رջ���K,��Һ©������������Һ�壬��ַ�Ӧ
�ܴ�B����ȫ��Ӧ����K��ͨ��һ�������
�ݳ���Eװ������Ϊ60.4 g
�ش��������⣺
��1��C��װ��ҩƷ�� ��E��ҩƷ�� ��F��ҩƷ������ ��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
��3����Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
��4����ʵ���к��ڲ�ͨ����������������Ʒ��̼���Ƶİٷֺ��� ���ƫС����ƫ����Ӱ�족��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
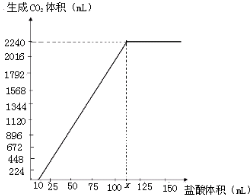 ��2012?բ����һģ��̼���ƺ�̼�������������������ж�����Ҫ��Ӧ�ã�����3.58g Na2CO3��NaHCO3������������Ƴ���Һ�߽������εμ�80.0mL 1.0mol/L���ᣬ��Ӧ��ȫ�����ɵ�CO2��������ɱ�״���µ����Ϊ896mL��������ȫ���ݳ�����ͬ������1��ͨ�������֪������Ӧ��
��2012?բ����һģ��̼���ƺ�̼�������������������ж�����Ҫ��Ӧ�ã�����3.58g Na2CO3��NaHCO3������������Ƴ���Һ�߽������εμ�80.0mL 1.0mol/L���ᣬ��Ӧ��ȫ�����ɵ�CO2��������ɱ�״���µ����Ϊ896mL��������ȫ���ݳ�����ͬ������1��ͨ�������֪������Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿������С�����ѧ ���ͣ�058
����̼�����ơ�̼���ƾ���(Na2CO3��xH2O)�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ��������ͼ��ʾװ�ý���ʵ�飮�ش��������⣺
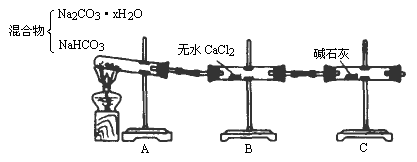
(1)Aװ�õ�������________��
(2)Bװ�õ�������________��
(3)Cװ�õ�������________��
(4)�����Թ�A��װ��̼�����ƺ�Na2CO3��xH2O�����3.7g���þƾ��Ƽ��ȵ���Ӧ��ȫ����ʱ�Թ�B��������1.89g��C����������0.22g����x��ֵΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ������һ�ν��Կ��Ի�ѧ�Ծ��������棩 ���ͣ�������
��13�֣�����̼�����ƺ�̼���ƹ������Ϊ�˲ⶨ�������̼���Ƶİٷֺ�������������װ�ã�
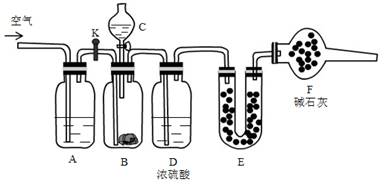
ʵ�鲽�裺
�ټ��װ��������
�ڽ�ҩƷװ�ã�����B��װ�� 9.5g��Ʒ��Eװ��ҩƷ����������56.0g
�۹رջ���K,��Һ©������������Һ�壬��ַ�Ӧ
�ܴ�B����ȫ��Ӧ����K��ͨ��һ�������
�ݳ���Eװ������Ϊ60.4 g
�ش��������⣺
��1��C��װ��ҩƷ�� ��E��ҩƷ�� ��F��ҩƷ������ ��
��2��A�з�����Ӧ�����ӷ���ʽΪ ��
��3����Ʒ��̼���Ƶ������ٷֺ���Ϊ ��
��4����ʵ���к��ڲ�ͨ����������������Ʒ��̼���Ƶİٷֺ��� ���ƫС����ƫ����Ӱ�족��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����̼�����ơ�̼���ƾ���(Na2CO3?xH2O)�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ��������ͼ��ʾװ�ý���ʵ�飬�ش��������⣺
�� Aװ�õ�������
�� Bװ�õ�������
�� Cװ�õ������� ��
������Ϊ��װ����ʲô����
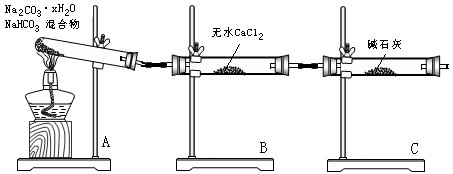
��5�������Թ�A��װ��̼�����ƺ�Na2CO3?xH2O�����33.4g���þƾ��Ƽ��ȵ���Ӧ��ȫ����ʱB����������15.3g��C����������2.2g����x��ֵΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com