
�����������������ʸֲĵ�����������ij���ߵ���Ҫ�ɷ�Ϊ����M��![]() ��������3.5%������������
��������3.5%������������![]() ��
��
��1��![]() Ԫ��ԭ�ӽṹʾ��ͼ ��
Ԫ��ԭ�ӽṹʾ��ͼ ��
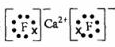
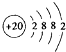
��2��![]() �����Ծ�ķǽ���Ԫ��A�γɻ�����D��D�ĵ���ʽΪ ��D�ķе��A��
�����Ծ�ķǽ���Ԫ��A�γɻ�����D��D�ĵ���ʽΪ ��D�ķе��A��![]() �γɵĻ�����E�ķе� ��
�γɵĻ�����E�ķе� ��
��3����ƽ�ø��������۵Ļ�ѧ����ʽ��
P+ FeO+ CaO![]()
![]() + Fe
+ Fe
��4����������������ϡ����������![]() ��Һ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
��Һ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ![]() .����
.����![]() �ķ�����____ _�������ӷ���ʽ�����
�ķ�����____ _�������ӷ���ʽ�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
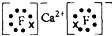
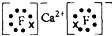
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

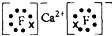
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣������������������ʸֲĵ�����������ij���ߵ���Ҫ�ɷ�Ϊ����M��![]() ��������3.5%������������
��������3.5%������������![]() ��
��
��1��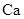 Ԫ�������ڱ���λ���� ����ԭ�ӽṹʾ��ͼ ��
Ԫ�������ڱ���λ���� ����ԭ�ӽṹʾ��ͼ ��
��2��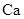 �����Ծ�ķǽ���Ԫ��A�γɻ�����D��D�ĵ���ʽΪ ��
�����Ծ�ķǽ���Ԫ��A�γɻ�����D��D�ĵ���ʽΪ ��
![]() ��3����ƽ�ø��������۵Ļ�ѧ����ʽ��
��3����ƽ�ø��������۵Ļ�ѧ����ʽ��
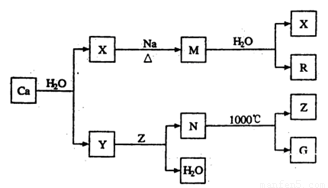
P + FeO+ CaO ���� Ca3(PO4)2+ Fe��
��4����������������ϡ���ᣬ�������![]() ��Һ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫM(OH)n������
��Һ�����ɰ�ɫ��״������Ѹ�ٱ�ɻ���ɫ������ɺ��ɫM(OH)n������![]() �ķ����� �������ӷ���ʽ�����
�ķ����� �������ӷ���ʽ�����
��5��ȡ1.6g������������ˮ��ַ�ӳ������224ml.H2����״������������Һ��ͨ��������CO2������ܵõ�CaCO3 g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ͽ��������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
������Ȼ�������㷺��IIA��Ԫ�أ����Ի�����N���ڡ��ӸƵ�����ʼ������һϵ�л�ѧ��Ӧ������ͼ��ʾ��
(1)��Ԫ�������ڱ���λ�ڵ�______���ڣ�Y�д��ڵĻ�ѧ������Ϊ_______________��N�Ļ�ѧʽ��______________��
��2��M��ˮ��Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
��3��Z��G�ĵ���ʽ�ֱ�Ϊ_______________________��_________________________.
��4��Z��R��Ӧ�������ɵ�����________________________________���ѧʽ����
��5��ʵ�������У�����NΪԭ���Ʊ����ʸƣ�����һ���Ʊ�������
_________________________________________________________________________.
��6�������������������ʸֲĵ�����������ij���ߵ���Ҫ�ɷֺ�FeԪ�غ�CaԪ�ء�
����ƽ�����ø����������Ļ�ѧ����ʽ��
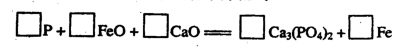
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com