
 �����������SO2�dz����Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�ѧ���о�����Ҫ���ݣ���������������и��⣺
�����������SO2�dz����Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�ѧ���о�����Ҫ���ݣ���������������и��⣺
| ||
| ||
| [N2]2?[H2O]2?[CO2]2 |
| [CH4]?[NO]4 |
| [N2]2?[H2O]2?[CO2]2 |
| [CH4]?[NO]4 |
| ||
| 1 |
| 2 |
| 0.8mol |
| 30L |
| 0.02667mol/L |
| 0.5min |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ���Ϻ���ʮ��У2012������ڶ���������ѧ���� ���ͣ�022
�����������SO2�dz����Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�ѧ���о�����Ҫ���ݣ���������������и��⣺
1���ü������ԭһ����������������һ�������Դ�������Ⱦ����Ӧ����ʽ���£�CH4(g)��4NO(g)![]() 2N2(g)��2H2O(g)��CO2(g)�÷�Ӧƽ�ⳣ������ʽΪ________��
2N2(g)��2H2O(g)��CO2(g)�÷�Ӧƽ�ⳣ������ʽΪ________��
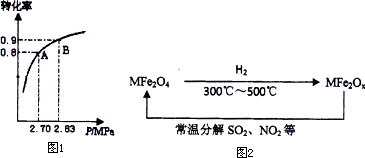
2�����������У��Ӵ����ڵķ�Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)��SO2��ƽ��ת��������ϵ��ѹǿ�Ĺ�ϵ��ͼ��ʾ��ij�¶��£���2.0 mol��SO2��1.0 mol��O2����30 L�����ܱ������У�30�뷴Ӧ��ƽ�����ϵ��ѹǿΪ2.70MPa����������ʾ�÷�Ӧ��ƽ��������________mol��L��1��min��1��
2SO3(g)��SO2��ƽ��ת��������ϵ��ѹǿ�Ĺ�ϵ��ͼ��ʾ��ij�¶��£���2.0 mol��SO2��1.0 mol��O2����30 L�����ܱ������У�30�뷴Ӧ��ƽ�����ϵ��ѹǿΪ2.70MPa����������ʾ�÷�Ӧ��ƽ��������________mol��L��1��min��1��
3����ͼƽ��״̬��A�䵽Bʱ���ı�����������________��
a���ּ���һЩ����
b���ּ���һЩ����
c����������ϵ���¶�
d�����������Ĵ���ѹ
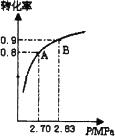
4���������ײ�����ȱλ������MFe2Ox�ڳ����£��ܽ������������SO2�ȷ����ֽ��ȥ��ת��������ͼ��ʾ����x��3.5��MΪZn����д��ZnFe2O3.5�ֽ�SO2�Ļ�ѧ����ʽ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����������SO2�dz����Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�ѧ���о�����Ҫ���ݣ���������������и��⣺
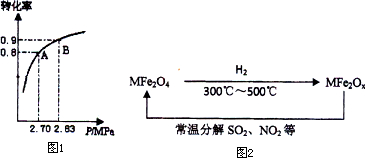
�����������SO2�dz����Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�ѧ���о�����Ҫ���ݣ���������������и��⣺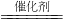 2SO3��g����SO2��ƽ��ת��������ϵ��ѹǿ�Ĺ�ϵ��ͼ1��ʾ��ij�¶��£���2.0mol SO2��1.0mol O2����30L�����ܱ������У�30�뷴Ӧ��ƽ�����ϵ��ѹǿΪ2.70MPa����������ʾ�÷�Ӧ��ƽ��������______mol?L-1?min-1��
2SO3��g����SO2��ƽ��ת��������ϵ��ѹǿ�Ĺ�ϵ��ͼ1��ʾ��ij�¶��£���2.0mol SO2��1.0mol O2����30L�����ܱ������У�30�뷴Ӧ��ƽ�����ϵ��ѹǿΪ2.70MPa����������ʾ�÷�Ӧ��ƽ��������______mol?L-1?min-1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�������������������һ����̼�Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
��1�������з�Ӧ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
S(g)��O2(g)��Ӧ����SO2(g)���Ȼ�ѧ����ʽΪ ��
![]() ��2��һ�������£�2SO2��g��+O2��g�� 2SO3��g������2L�ܱ�������ͨ��
��2��һ�������£�2SO2��g��+O2��g�� 2SO3��g������2L�ܱ�������ͨ��
2 mol SO2��g����1 mol O2��g����0.2 mol SO3��g����2 min��Ӧ�ﵽƽ��ʱ�����SO2�����ʵ���Ϊ1 mol�������£������������ټ���2 mol SO2��g���������´ﵽƽ��ʱSO2����ת���� 50%��ѡ���������������������
![]() ��3����һ�ܱ������з�����Ӧ2NO2 2NO+O2����Ӧ������NO2��Ũ����ʱ��仯
��3����һ�ܱ������з�����Ӧ2NO2 2NO+O2����Ӧ������NO2��Ũ����ʱ��仯
 ���鱨������ͼ��ʾ����ش�
���鱨������ͼ��ʾ����ش�
��������A����Ӧ��ǰ3min��������ƽ����Ӧ����Ϊ ��
��������A��B�ֱ��ʾ���Ǹ÷�Ӧ�ڱ���������������ʱ��ֻ�ı�����һ���������ʱ�ı仯��������������
���Ũ�ȡ�����ѹǿ�������¶ȡ���������
![]() ��4����ҵ��һ�����ܱ������в������з�Ӧ�ϳɼ״���CO(g)+2H2(g) CH3OH(g)
��4����ҵ��һ�����ܱ������в������з�Ӧ�ϳɼ״���CO(g)+2H2(g) CH3OH(g)
ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ��ʱ���c(CO)=0.l mol·L -1��
�ٸ÷�Ӧ��ƽ�ⳣ��K= ��
���������������������£����������ѹ����ԭ����1/2����ԭƽ����ȣ�������
��˵����ȷ���� ������ţ���
a��������Ũ�ȼ��� b������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c���״������ʵ������� d������ƽ��ʱn(H2) / n(CH3OH)����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com