
��1��Ц������������Ӧ����ҽ�Ƶ�������֮һ��������NH4NO3���������·ֽ�����������N2O���һ�֣��˷�Ӧ�Ļ�ѧ����ʽΪ____________________________���������ת�Ƶķ������Ŀ�����й�������ΪN2O��CO2���Ӿ������ƵĽṹ����������ʽ��������ռ乹����_____________�Σ�N2OΪ_____________������ԡ��Ǽ��ԡ������ӡ�
��2����һ�ֳ��������������ȷ£����治�����������������綾������COCl2��2CHCl3+O2![]() 2HCl+2COCl2��Ϊ�˷�ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ���ʵ��Լ��ǣ� ��
2HCl+2COCl2��Ϊ�˷�ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ���ʵ��Լ��ǣ� ��
A.����KI��Һ B.NaOH��Һ
C.��̪��Һ D.�����ữ��AgNO3��Һ
��3����Ϊ�������������ҽѧ��Ҳ�������ӣ��������ϸ���������֬�����ϸ�������������ͣ��Ӷ�ʹ��ĩ��������ʱֹͣ�������������������Ϊ80%�������20%��������ɵĻ��������Ϊ�����õ������������Ԫ�����ڱ���λ�ڵ�____________���ڣ���____________�壬����������ƽ����Է�������Ϊ____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
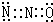
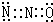
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и����ڶ���ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
�������ķ��ֺ�ʹ�ã���������ʷ���˲����һ��ɾͣ�����ʹ�����ڽ�������ʱ�о�������ʹ��
��1����Ц����(N2O)����������Ӧ����ҽ�Ƶ�������֮һ���й�������ΪN2O��CO2�������ƵĽṹ(��������ʽ)����֪N2O��������ԭ��ֻ��һ����ԭ����������N2O�ĵ���ʽ�ɱ�ʾΪ________����ռ乹����________�ͣ��ɴ˿ɼ�����________����(����ԡ��Ǽ��ԡ�)
��2����һ�ֳ����������ȷ£����治�����������������綾�����(COCl2)��2CHCl3��O2��2HCl��2COCl2��Ϊ��ֹ�ж��¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ���ʵ��Լ���________��
A����̪��Һ B�����۵⻯����Һ C��NaOH��Һ D��������ϡ��Һ
��3����Ϊ�������������ҽѧ�ϱ������ӣ��������ϸ���ʵ���֬�����ϸ�������������ͣ��Ӷ�ʹ��ĩ��������ʱֹͣ�������������������Ϊ80%�����20%������ɵĻ�����壬��Ϊ�����õ���������ϡ������믵Ļ�ѧ���ʲ����ã�������һ�������¿����������ʷ�Ӧ���ɻ������ȡ1mol�����3.5mol�������ܱ������У���һ��������ʹ��Ӧ��ϣ�ʣ����1.5mol������ͬʱ�а�ɫ�������ɣ��˰�ɫ����Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��У�����ڶ������������ۣ���ѧ���� ���ͣ������
[��ѧ����ѡ��ѧ������]��15�֣��������ķ��ֺ�ʹ�ã�������ҽ��ʷ���˲����һ��ɾͣ�����ʹ�����ڽ�������ʱ�о�������ʹ��
��1��Ц������������Ӧ����ҽ�Ƶ�������֮һ��������NH4NO3���������·ֽ�����������N2O���һ�֣��˷�Ӧ�Ļ�ѧ����ʽΪ______________________________��N2OҲ����ͨ��ǿ��ԭ�Խ�������Mg����ϡHNO3��Ӧ�Ƶã�д���÷�Ӧ�����ӷ���ʽ________________________________���й�������ΪN2O��CO2�־������ƵĽṹ����������ʽ������֪N2O��������ԭ��ֻ��һ����ԭ����������N2O�ĵ���ʽ�ɱ�ʾΪ_____________��
��2����һ�ֳ����������ȷ£����治�����������������綾�������COCl2����
 ��Ϊ�˷�ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ����
��Ϊ�˷�ֹ�¹ʣ�ʹ��ǰ�����ڼ����ȷ��Ƿ����
���Լ���________________��
����A������һ�⻯����Һ��B��NaOH��ҺC����̪��Һ D�������ữ��AgNO3��Һ
��3������³������������һ�־ֲ����������������ýϿ죬��ǿ�����Խϵͣ���ṹ
��ʽΪ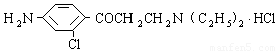 ���������Լױ�Ϊԭ�Ϻϳ�
���������Լױ�Ϊԭ�Ϻϳ�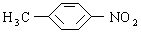 ���䷴Ӧ����ʽΪ______________________________________���پ���________________��Ӧ���Ӧ���ͣ����ϳ�
���䷴Ӧ����ʽΪ______________________________________���پ���________________��Ӧ���Ӧ���ͣ����ϳ�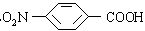 ���پ���һϵ�з�Ӧ���ϳɡ�
���پ���һϵ�з�Ӧ���ϳɡ�
��4����Ϊ�������������ҽѧ��Ҳ�������ӣ��������ϸ���ʵ���֬�����ϸ�������������ͣ��Ӷ�ʹ��ĩ��������ʱֹͣ�������������������Ϊ80%�������20%��������ɵĻ������Ϊ�����õ������������Ԫ�����ڱ���λ��___________���ڣ�________�壬����������ƽ��ʽ��Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com