
�����Ǹ������Դ���⣬��ˮ��Դ�����þ��зdz������ķ�չǰ������ˮ����Ԫ����Br-��ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ�����������ͼ��
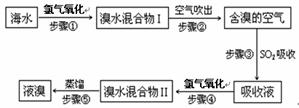
��1������ٷ�Ӧ�����ӷ���ʽΪ ��
��2������۷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��Br��ԭ�������� �������ڱ���λ�ڵ� ���ڡ��� �塣
��4�����������Ĺ����У��¶�Ӧ������80~90�档�¶ȹ�����Ͷ������������������ԭ�� ��
��5��Ϊʲô��ֱ���á���ˮ�����I����Ҫ�á���ˮ�����II���������õ�Һ��?
��
��1��2Br-��Cl2��Br2��2Cl- (3��)
����2��SO2��Br2��2H2O��2HBr��H2SO4 (3��)
����3��35(1��) ����(1��) ��VIIA (1��)
����4���¶ȹ��ߣ�����ˮ�������������ų�����������ˮ���ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ͡�(3��)
����5������ˮ�����I������Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ�����������������ɱ��ߣ�����������������SO2���ա��������Ȳ���ʵ�����ǽ���ˮŨ���ˡ�(3��)


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
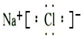
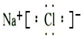
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�γ���ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��14�֣�������һ�������Դ���⡣����Ӧ�ú��磬�������ȼҵ��ԭ�ϡ�
��1�����ᴿ���ʳ������20%��NaCl��Һ��Ӧѡ�õ�������__________��ѡ����ţ���
a. ��ƿ b. ����ƿ c. ��Ͳ d. ��ͷ�ι�
��2����ⱥ��ʳ��ˮ���ø�Ĥ���ۻ�����Ĥ���ۡ�ͼ7Ϊ�����ӽ���Ĥ���ۣ�ֻ����������ͨ����ʾ��ͼ��
�����ж�EΪ_________����
���Ƶõ��ռ���Һ��_______��������ĸ��������
���Ƶõ��ռ���Һ����������NaCl���������к���Cl���ľ�������ǣ�______________________________________________________________________��
��3��20��ʱ�Ƶ��ռ���Һ�У�����NaOH�����ı仯��NaCl�ﵽ����״̬ʱ���������������ı仯������ͼ������20��ʱ����������B����ʾ����Һ���ɲ���______________
��������NaCl�ĺ������ﵽ�ᴿĿ�ģ���������_________________________________________________________________��
��4�����ⶨij�����ռ���Һ��Ʒ����֪�ܶ�Ϊ��g��cm��3����NaOH�ĺ������ɲ��õķ�����___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com