



 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ʷ��ӵĴ�С������ | B��Һ̬���ʷ��ӵĴ�С������ |
| C���������ʷ��ӵĴ�С������ | D���������ʷ��ӵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��5.85gNaCl��������100gˮ��������Һ���ʵ���Ũ�Ȳ�����1mol/L |
| B��20gŨ��Ϊc mol��L-1�İ�ˮ�м���һ������ˮϡ�ͳ�0.5cmol��L-1�������ˮ�����С��20ml |
| C��ij�Ȼ�þ��Һ���ܶ�Ϊ1.18 g��cm��3������þ���ӵ���������Ϊ5.1%,300 mL����Һ��Cl�������ʵ���Լ����1.50 mol |
D��V L Fe2(SO4)3��Һ�к�Fe3��m g������Һ��SO42-�����ʵ���Ũ��Ϊ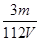 mol��L��1 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��15g��(��CH3)�����еĵ�������NA |
| B�����³�ѹ�£�4g���������е�ԭ����ĿΪNA |
| C����״���£�1L������ȼ�պ����ɵ���̬����ķ�����Ϊ6/22.4 NA |
| D�����³�ѹ�£�1mol���������еĹ��ۼ���ĿΪ8NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | KCl | K2SO4 | ZnSO4 | ZnCl2 |
| 1 | 0��3mol | 0��2mol | 0��1mol | �� |
| 2 | 0��1mol | 0��3mol | �� | 0��1mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| 18K��ɷ� | Au | Ag | Cu |
| ���������������� | 75��00% | _________ | _________ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com