
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
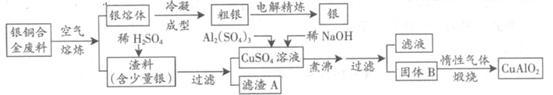
��ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450���80�棩
��1����⾫����ʱ��������ӦʽΪ ������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ����ʽΪ ��
��2����������B�����Ϊ �������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ ��
��3��������չ�����һ����Ӧ�Ļ�ѧ����ʽ�� CuO+ Al2O3 CuAlO2 + ����
CuAlO2 + ����
��4������ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0kg�����е�ͭ����ȫת��Ϊ mol CuAlO2��������Ҫ1.0mol?L��1��Al2(SO4)3��Һ L��
��5��CuSO4��ҺҲ�������Ʊ������������������ �����ˡ�ϴ�Ӻ��
��1��Ag++e��==Ag��1�֣� 2NO+O2==2NO2��2�֣�
��2��Al(OH)3��CuO��2�֣� Al(OH)3+OH��==AlO2��+2H2O��2�֣�
��3��4 2 4 O2����2�֣�
��4��50��2�֣� 25��2�֣�
��5������Ũ������ȴ�ᾧ��2�֣�
��������
��1����⾫��ͭʱ����ͭ����������ͭ������������ͭ��Һ�����Һ����⾫����������ƣ���������������������������������Һ�����Һ��������Ҫ��ӦʽΪAg��e��=Ag+��������ӦʽΪAg++e��=Ag�����ǽ����˳�������������������ϡ���ᣬ��Ϊϡ�����Ƿ��������ᣬ������A����Ҫ�ɷ���Ag��ϡ�������������ᣬ���ܽ�����������ɫ���壬��3Ag+4HNO3(ϡ) =3AgNO3+NO��+2H2O����ɫ��һ�����������ڿ���Ѹ�ٱ�����Ϊ����ɫ�Ķ����������壬��2NO+O2=2NO2��
��2��CuAlO2����Ϊ��2�ۣ���Ϊ+3�ۣ���ͭΪ+1�ۣ�����仯ѧʽҲ����дΪCu2O?Al2O3���൱��������ͭ�������������ʵ���֮��Ϊ1��1�ۺ���һ���ɴ����ƣ�����B����Ҫ�ɷ������ʵ���֮��Ϊ1��1��Al(OH)3��CuO����ΪCu(OH)2��ʼ�ֽ���¶�Ϊ80�棬ˮ�ķе����80�棬�����ǰ�����ķ�ӦΪAl3++3OH��=Al(OH)3����Cu2++2OH��==Cu(OH)2����Cu(OH)2 CuO+H2O��Al(OH)3��ʼ�ֽ���¶�Ϊ450�棬�ڶ�������������ʱ�����ķ�ӦΪ2Al(OH)3
CuO+H2O��Al(OH)3��ʼ�ֽ���¶�Ϊ450�棬�ڶ�������������ʱ�����ķ�ӦΪ2Al(OH)3 Al2O3+3H2O��4CuO
Al2O3+3H2O��4CuO 2Cu2O+O2�������������������������NaOH��ǿ���ǿ����Һ�������������NaOH���ܽⲿ�ֻ�ȫ�����������������ӷ���ʽΪAl(OH)3+OH��=AlO2��+2H2O����ؽ�����Ŀ���������ļ��٣�
2Cu2O+O2�������������������������NaOH��ǿ���ǿ����Һ�������������NaOH���ܽⲿ�ֻ�ȫ�����������������ӷ���ʽΪAl(OH)3+OH��=AlO2��+2H2O����ؽ�����Ŀ���������ļ��٣�
��3������������ԭ��Ӧ�������ƶϣ�ͭԪ����+2�۽�Ϊ+1�ۣ���Ԫ�ػ��ϼ۲��䣬�ɴ��ƶϷ�Ӧǰ������Ԫ��һ���������һ��ϼ��ɡ�2����Ϊ���ڵ�0�ۣ���ȱ�ٵ�������Ϊ���������ݻ��ϼ���������ƽ�ɵã�4CuO+2Al2O3 4CuAlO2+O2�������߸�����1����ƽ�����跴Ӧ������������ϵ��Ϊ1���������غ�ɵ�������CuAlO2��ϵ��Ϊ2������ͭ�غ�ɵ÷�Ӧ��CuO��ϵ��Ϊ2���������غ�ɵ�������ϵ��Ϊ1/2����2CuO+1Al2O3
4CuAlO2+O2�������߸�����1����ƽ�����跴Ӧ������������ϵ��Ϊ1���������غ�ɵ�������CuAlO2��ϵ��Ϊ2������ͭ�غ�ɵ÷�Ӧ��CuO��ϵ��Ϊ2���������غ�ɵ�������ϵ��Ϊ1/2����2CuO+1Al2O3 2CuAlO2+1/2O2����ϵ���ӱ��ɵõ�4CuO+2Al2O3
2CuAlO2+1/2O2����ϵ���ӱ��ɵõ�4CuO+2Al2O3 4CuAlO2+O2������
4CuAlO2+O2������
��4��5.0kg=5.0��103g����ͭ�Ͻ������ͭ������Ϊ5.0��103g��63.5%��ͭԪ�ص����ԭ������Ϊ63.5����m/M=n����ͭ�����ʵ���Ϊ5.0��103g��63.5%��63.5g/mol=50mol������ͭ�غ�ɵ�ת����ϵʽ��Cu��CuAlO2������CuAlO2��Cu�����ʵ���֮�ȵ���ϵ��֮�ȣ���ͭ��ȫת�����Ա�Ϊ50mol CuAlO2���������غ�ɵ�ת����ϵʽ��Al2(SO4)3��2CuAlO2������Al2(SO4)3��CuAlO2�����ʵ���֮�ȵ���ϵ��֮�ȣ���������Ҫ25mol Al2(SO4)3����V=n/c����������Ҫ��������Һ�����Ϊ25mol��1.0mol/L=25L��
��5�������Ļ�ѧʽΪCuSO4?5H2O��������ͭ��Һ�ᾧ�����Ľᾧˮ��������ݻ���������ᴿ�ķ����ƶϣ�������ͭ��Һ�еõ������Ļ�������������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӻ��
�����㶨λ�������Դ��и�����л��������Ʊ�ͭ������Ʒ�Ĺ���Ϊ��������ϸ����Ĺ�������ͼ�������ʣ���Ҫ�����˿����Գ����������ǽ���Ԫ�ؼ��仯�������Ҫ���ʵ����գ��Գ�����ѧ����ʽ�����ӷ���ʽ����д���Ե��ص���ɺ͵����̵����⣬�Լ��Թ�������ͼ�Ľ�������ṩ���ݵ�Ӧ�õȣ����Դ˴ﵽ���鿼��Ӧ�û���֪ʶ�����ѧ�����������ͨ����ƵĹ�������ͼ���ʣ����ؿ��鿼����Ag��Cu��Al��N��Ԫ�ص���ػ��������ʵ����ճ̶ȣ����鿼�������ʵ�����Ħ�����������������ʵ���Ũ�ȵȸ�����˽⣻���鿼���������غ㶨�ɵ��˽⣻���鿼���������ʵ��������������ʵ���Ũ��֮������ϵ�����йؼ�������������鿼��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȼ���ʵ����������ա�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
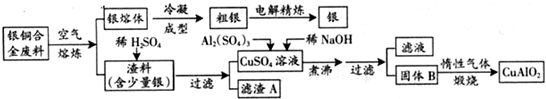
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
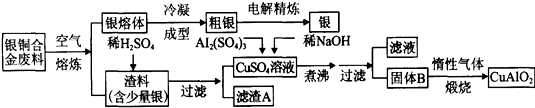
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
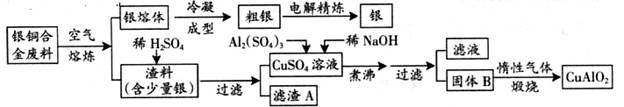
���� ��ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450���80�棩
��1����⾫����ʱ��������ӦʽΪ���� ������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ����ʽΪ������������������������������������ ��
��2����������B�����Ϊ������������������������ �������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ������������������������������ ��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ�� CuO + ��Al2O3�� �� �� CuAlO2 + �� ��
��4������ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0kg�����е�ͭ����ȫת��Ϊ�� mol CuAlO2��������Ҫ1.0mol��L-1��Al2(SO4)3��Һ������ L��
(5)CuSO4��ҺҲ�������Ʊ�������������������������������������� �����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ���㶫���������� ���ͣ������
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�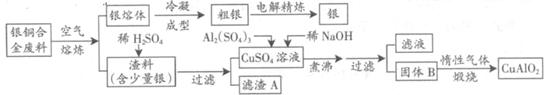
��ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450���80�棩
��1����⾫����ʱ��������ӦʽΪ ������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ����ʽΪ ��
��2����������B�����Ϊ �������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ ��
��3��������չ�����һ����Ӧ�Ļ�ѧ����ʽ�� CuO+ Al2O3 CuAlO2 + ����
CuAlO2 + ����
��4������ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0kg�����е�ͭ����ȫת��Ϊ mol CuAlO2��������Ҫ1.0mol?L��1��Al2(SO4)3��Һ L��
��5��CuSO4��ҺҲ�������Ʊ������������������ �����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡԭ��У���˸�����ѧ�ڵ�һ�������Ի�ѧ�Ծ��������棩 ���ͣ������
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ�������ͼ��
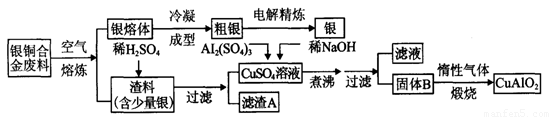
��1����⾫����ʱ��������ӦʽΪ_______________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���ú���ɫ������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2����������B�����Ϊ_____________�������ɹ���B�Ĺ����У��������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ_____________________��
��3�����չ��������ɵ�����������NH3�ڴ��������·�Ӧ�Ļ�ѧ����ʽΪ_____________________�������Ӧ�л��а��̲������ð���Ϊ______________��
��4������ͭ�Ͻ���ͭ����������Ϊ64����������3��0kg�����е�ͭ����ȫת��Ϊ__________molCuAlO2��������Ҫ1��0 mol��L��1��Al2(SO4)3��Һ___________L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com