
(1)3.320 g��������Cԭ�ӵ����ʵ���Ϊ___________��
(2)��ʽ����3.320 g��������H��Oԭ�ӵ����ʵ�����
(3)�������ʵ��ʽΪ___________��
(4)д������������������Է���������С�ķ���������Ŀ��ܵĽṹ��ʽ��
������(1)��Ӧ�������ΪCO2��O2�Ļ�����壬ͨ��Na2O2�����������1.792 L����2Na2O2+2CO2![]() 2Na2CO3+O2�У�
2Na2CO3+O2�У�
n(CO2)=![]() =0.160 mol��
=0.160 mol��
(2)��ȼ��ͨʽ
CxHyOz+(![]() )O2
)O2![]() xCO2+
xCO2+![]() H2O
H2O
�ɵ�3.320 g��������
n(C)=n(CO2)=0.160 mol��
��![]() n(O)-
n(O)-![]() n(H)=
n(H)=![]() =0.010 mol��
=0.010 mol��
��0.160 mol��12 g/mol+n(H)��1 g/mol+n(O)��16 g/mol=3.320 g��
���n(H)=0.120 mol��n(O)=0.080 mol��
(3)n(C)��n(H)��n(O)=0.160 mol��0.120 mol��0.080 mol=4��3��2��
��������ʵ��ʽΪC4H3O2��
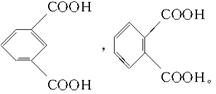
(4)��Է���������С�ķ������ᣬ����Ӧ����һ���������Ӷ���ȷ��������ķ���ʽΪC8H6O4�����������ֻ���Ȼ����������������ţ���Ӧ��2����COOH�����ڡ��䡢������ͬ���칹�塣
�𰸣�(1)0.160 mol (2)H��0.120 mol O��0.080 mol (3)C4H3O2
![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ȫ����Ʊ�����ѧ�ս̰� �ս̰� ���ͣ�022
ȡ3.320 gֻ���Ȼ����������������ŵĹ�̬������������4.00 L�����У��������ȼ��������ȫȼ�գ���Ӧ�������������0.224 L��������ͨ��������Na2O2���������1.792 L(����������ڱ�״���²ⶨ)����ش�
(1)3.320 g��������Cԭ�ӵ����ʵ���Ϊ________��
(2)��ʽ����3.320 g��������H��Oԭ�ӵ����ʵ�����
(3)�������ʵ��ʽΪ________��
(4)д������������������Է���������С�ķ���������Ŀ��ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���̲���ȫ��������л�ѧ��2�������ޣ�����³�ư�α�棩 ³�ư�α�� ���ͣ�038
ȡ3.320 gֻ���Ȼ����������������ŵĹ�̬��������������4.00 L�����У��������ȼ��������ȫȼ�գ���Ӧ�������������0.224 L��������ͨ��������Na2O2���������1.792 L(����������ڱ�״���²ⶨ)����ش�
(1)3.320 g��������Cԭ�ӵ����ʵ���Ϊ________��
(2)��ʽ����3.320 g��������H��Oԭ�ӵ����ʵ�����
(3)�������ʵ��ʽΪ________��
(4)д������������������Է���������С�ķ���������Ŀ��ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ȫ��ָ����ѧ�ս̰� �ս̰� ���ͣ�038
ȡ3.320 gֻ���Ȼ����������������ŵĹ�̬������������4.00 L�����У��������ȼ��������ȫȼ�գ���Ӧ�������������0.224 L��������ͨ��������Na2O2���������1.792 L(����������ڱ�״���²ⶨ)����ش�
(1)3.320 g��������Cԭ�ӵ����ʵ���Ϊ________��
(2)��ʽ����3.320 g��������H��Oԭ�ӵ����ʵ�����
(3)�������ʵ��ʽΪ________��
(4)д������������������Է���������С�ķ���������Ŀ��ܵĽṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡ3.320 gֻ���Ȼ����������������ŵĹ�̬��������������4.00 LO2�У�����ȼ������ȫȼ�ա���Ӧ�������������0.224 L��������ͨ�������Ĺ���Na2O2���������1.792 L(����������ڱ�״���²ⶨ)����ش�
(1)3.320 g��������̼ԭ�ӵ����ʵ���Ϊ______��
(2)��ʽ����3.320 g���������⡢��ԭ�ӵ����ʵ���H______mol��O______mol��
(3)��������C��H��O��ԭ����֮��Ϊ______��
(4)д���������ϸ���������Է���������С�ķ���������Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com