
| |||||||||||||||||||
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2008?�㶫��þ��ͭ�Ƚ��������������ڶ���ø�ĸ����ӣ���ҵ�ϴӺ�ˮ����ȡþʱ�����Ʊ���ˮ�Ȼ�þ��Ȼ�������ڵ�⣬�õ�����þ��
��2008?�㶫��þ��ͭ�Ƚ��������������ڶ���ø�ĸ����ӣ���ҵ�ϴӺ�ˮ����ȡþʱ�����Ʊ���ˮ�Ȼ�þ��Ȼ�������ڵ�⣬�õ�����þ��| ������ | NaF | MgF2 | SiF4 |
| �۵�/K | 1266 | 1534 | 183 |
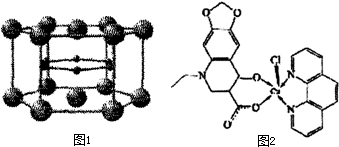
 ��Cu��I����I��ʾ���ϼ�Ϊ+1��ʱ���ֱ��γ�a��b��
��Cu��I����I��ʾ���ϼ�Ϊ+1��ʱ���ֱ��γ�a��b��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| MgO | Al2O3 | MgCl2 | AlCl3 | |
| �۵�/�� | 2852 | 2072 | 714 | 190��2.5��105Pa�� |
| �е�/�� | 3600 | 2980 | 1412 | 182.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�ij���������У��һ��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪MgO��MgCl2���۵�ֱ�Ϊ2800�桢604�棬��MgO��MgCl2�������ں�ͨ���⣬���ɵõ�����þ����ˮ�к���MgCl2����ҵ�ϴӺ�ˮ����ȡþ����ȷ�ķ����� �� ��
A����ˮ Mg(OH)2
Mg(OH)2 Mg
Mg
B����ˮ MgCl2��Һ
MgCl2��Һ MgCl2����
MgCl2���� Mg
Mg
C����ˮ Mg(OH)2
Mg(OH)2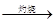 MgO
MgO Mg
Mg
D����ˮ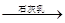 Mg(OH)2
Mg(OH)2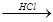 MgCl2��Һ
MgCl2��Һ
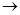 MgCl2����
MgCl2���� Mg
Mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
��֪MgO��MgCl2���۵�ֱ�Ϊ2800�桢604�棬��MgO��MgCl2�������ں�ͨ���⣬���ɵõ�����þ����ˮ�к���MgCl2����ҵ�ϴӺ�ˮ����ȡþ��������ķ�����
A�� ��ˮ Mg(OH)2
Mg(OH)2 Mg
Mg
B�� ��ˮ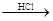 MgCl2��Һ
MgCl2��Һ MgCl2����
MgCl2���� Mg
Mg
C�� ��ˮ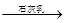 Mg(OH)2
Mg(OH)2 MgO
MgO Mg
Mg
D����ˮ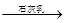 Mg(OH)2
Mg(OH)2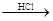 MgCl2��Һ
MgCl2��Һ
 MgCl2����
MgCl2���� Mg
Mg
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com