
��12�֣���������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�������ͼ��װ�á�

��1����Ƭ�ϵĵ缫��ӦʽΪ ��ͭƬ�ϵĵ缫��ӦʽΪ ��ͭƬ��Χ��Һ����� ������
��2����2 min������Ƭ��ͭƬ֮���������Ϊ1.2g�������������ĵ���Ϊ mo1���ö�ʱ����������ͭ��ʾ��ƽ����Ӧ����Ϊ ��
��3��������װ�ø�Ϊ����ͼ��ʾ��װ��Ҳ�ܴﵽ��ԭװ����ͬ�����ã�ͬʱ�ܱ�ֹ֤ͣʹ�ø�װ��ʱ��Ӧ�ﲻ�����KCl��Һ��ͨ������Һ�γɱպϻ�·�����ã�������ͭ��ҺӦ��ע�� �����ࡱ�����Ҳࡱ�����ࡱ���ձ��У���2 min����Ƭ������2.8g�����м�U�ι���K+�������� mo1/min�������Ͼ����跴Ӧ��������Һ������䣩��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
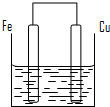 ��������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�����ͼ��װ�ã�
��������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�����ͼ��װ�ã�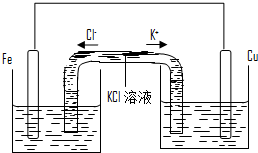
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�������ͼ��װ�á�
��1����Ƭ�ϵĵ缫��ӦʽΪ ��ͭƬ�ϵĵ缫��ӦʽΪ ��ͭƬ��Χ��Һ����� ������
��2����2 min������Ƭ��ͭƬ֮���������Ϊ1.2g�������������ĵ���Ϊ mo1���ö�ʱ����������ͭ��ʾ��ƽ����Ӧ����Ϊ ��
��3��������װ�ø�Ϊ����ͼ��ʾ��װ��Ҳ�ܴﵽ��ԭװ����ͬ�����ã�ͬʱ�ܱ�ֹ֤ͣʹ�ø�װ��ʱ��Ӧ�ﲻ��ģ�KCl��Һ��ͨ������Һ�γɱպϻ�·�����ã�������ͭ��ҺӦ��ע�� �����ࡱ�����Ҳࡱ�����ࡱ���ձ��У���2 min����Ƭ������2.8g�����м�U�ι���K+�������� mo1/min�������Ͼ����跴Ӧ��������Һ������䣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ����һ�и�һ��ѧ�ڵ����ζο���ѧ�Ծ� ���ͣ������
��12�֣���������ȵ���Ƭ��ͭƬ�õ�����������500mL����ͭ��Һ�й�������ͼ��װ�á�
��1����Ƭ�ϵĵ缫��ӦʽΪ ��ͭƬ�ϵĵ缫��ӦʽΪ ��ͭƬ��Χ��Һ����� ������
��2����2 min������Ƭ��ͭƬ֮���������Ϊ1.2g�������������ĵ���Ϊ mo1���ö�ʱ����������ͭ��ʾ��ƽ����Ӧ����Ϊ ��
��3��������װ�ø�Ϊ����ͼ��ʾ��װ��Ҳ�ܴﵽ��ԭװ����ͬ�����ã�ͬʱ�ܱ�ֹ֤ͣʹ�ø�װ��ʱ��Ӧ�ﲻ��ģ�KCl��Һ��ͨ������Һ�γɱպϻ�·�����ã�������ͭ��ҺӦ��ע�� �����ࡱ�����Ҳࡱ�����ࡱ���ձ��У���2 min����Ƭ������2.8g�����м�U�ι���K+�������� mo1/min�������Ͼ����跴Ӧ��������Һ������䣩��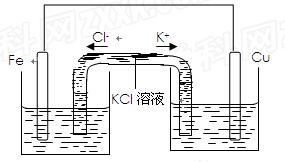
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�γ���ѧ��һ���£����л�ѧ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com